Hispidin: Difference between revisions
Appearance
Content deleted Content added
m Journal cites:, added 1 PMID, completed 1 page range using AWB (10473) |
m GHS update: remove empty EUClass/Rphrase/Sphrase parameters (depr) |
||
| (12 intermediate revisions by 10 users not shown) | |||
| Line 1: | Line 1: | ||
{{ |
{{Chembox |
||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Watchedfields = changed |
| Watchedfields = changed |
||
| verifiedrevid = 437982353 |
| verifiedrevid = 437982353 |
||
| Name = Hispidin |
| Name = Hispidin |
||
| ImageFile = Hispidin. |
| ImageFile = Hispidin.svg |
||
| ImageSize = 200px |
|||
| ImageName = Chemical structure of hispidin |
| ImageName = Chemical structure of hispidin |
||
| ImageAlt = Chemical structure of hispidin |
| ImageAlt = Chemical structure of hispidin |
||
| |
| PIN = 6-[(1''E'')-2-(3,4-Dihydroxyphenyl)ethen-1-yl]-4-hydroxy-2''H''-pyran-2-one |
||
| OtherNames = 6-(3,4- |
| OtherNames = 6-(3,4-Dihydroxystyryl)-4-hydroxy-2-pyrone |
||
|Section1= |
|Section1={{Chembox Identifiers |
||
| CASNo = 555-55-5 |
| CASNo = 555-55-5 |
||
| CASNo_Ref = {{cascite|correct|??}} |
| CASNo_Ref = {{cascite|correct|??}} |
||
| |
| CASNoOther = |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| ⚫ | |||
| UNII = SSJ18CG55E |
|||
| ⚫ | |||
| SMILES = C1=CC(=C(C=C1C=CC2=CC(=O)C=C(O2)O)O)O |
| SMILES = C1=CC(=C(C=C1C=CC2=CC(=O)C=C(O2)O)O)O |
||
| |
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
||
| ChemSpiderID = 13975015 |
| ChemSpiderID = 13975015 |
||
| |
| SMILES2 = OC=2/C=C(/C=C/c1ccc(O)c(O)c1)OC(=O)C=2 |
||
| ⚫ | |||
| InChI = 1/C13H10O5/c14-9-6-10(18-13(17)7-9)3-1-8-2-4-11(15)12(16)5-8/h1-7,14-16H/b3-1+ |
|||
| InChIKey = SGJNQVTUYXCBKH-HNQUOIGGBX |
|||
| ⚫ | |||
| StdInChI = 1S/C13H10O5/c14-9-6-10(18-13(17)7-9)3-1-8-2-4-11(15)12(16)5-8/h1-7,14-16H/b3-1+ |
| StdInChI = 1S/C13H10O5/c14-9-6-10(18-13(17)7-9)3-1-8-2-4-11(15)12(16)5-8/h1-7,14-16H/b3-1+ |
||
| |
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
||
| StdInChIKey = SGJNQVTUYXCBKH-HNQUOIGGSA-N |
| StdInChIKey = SGJNQVTUYXCBKH-HNQUOIGGSA-N |
||
}} |
}} |
||
| |
|Section2={{Chembox Properties |
||
| |
| C=13| H=10 | O=5 |
||
| Appearance = |
| Appearance = |
||
| Density = |
| Density = |
||
| MeltingPt = |
| MeltingPt = |
||
| BoilingPt = |
| BoilingPt = |
||
| Solubility = |
| Solubility = |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| AutoignitionPt = |
|||
| GHS_ref=<!-- no GHS data in PubChem Dec2021 --> |
|||
| ⚫ | |||
}} |
}} |
||
| ⚫ | '''Hispidin''' is a natural substance. It can also be synthesized.<ref>{{Cite journal |pmid=9088624 |doi=10.1023/A:1007321227010 |year=1997 |last1=Gonindard |first1=C. |last2=Bergonz i|first2=C. |last3=Denier |first3=C. |last4=Sergheraert |first4=C. |last5=Klaebe |first5=A. |last6=Chavant |first6=L. |last7=Hollande |first7=E. |journal=Cell Biology and Toxicology |volume=13 |issue=3 |pages=141–53 |title=Synthetic hispidin, a PKC inhibitor, is more cytotoxic toward cancer cells than normal cells in vitro|s2cid=755744 }}</ref> |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| Autoignition = |
|||
| RPhrases = <!-- {{R10}}, {{R23}}, {{R34}}, {{R50}} etc. --> |
|||
| SPhrases = <!-- {{S1/2}}, {{S9}}, {{S16}}, {{S26}}, {{S36/37/39}}, {{S45}}, {{S61}} etc. --> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | '''Hispidin''' is a natural substance. It can also be |
||
Hispidin 4-O-β- |
Hispidin 4-''O''-β-<small>D</small>-glucopyranoside can be found in ''[[Pteris ensiformis]]''<ref>{{cite journal | doi = 10.1016/j.foodchem.2007.03.055 | title = Identification of phenolic antioxidants from Sword Brake fern (Pteris ensiformis Burm.) | journal = Food Chemistry | volume = 105 | pages = 48–56 | year = 2007 | last1 = Chen | first1 = Y. | last2 = Chang | first2 = F. | last3 = Lin | first3 = Y. | last4 = Wang | first4 = L. | last5 = Chen | first5 = J. | last6 = Wu | first6 = Y. | last7 = Wu | first7 = M. }}</ref> whereas hispidin derivatives, such as [[phellibaumin]]s, can be found in the edible mushroom ''[[Inonotus xeranticus]]''<ref>{{cite journal | doi = 10.1021/np050453n| pmid = 16499338| title = Hispidin Derivatives from the Mushroom ''Inonotusxeranticusand'' Their Antioxidant Activity| journal = Journal of Natural Products| volume = 69| issue = 2| pages = 299–301| year = 2006| last1 = Lee| first1 = In-Kyoung| last2 = Seok| first2 = Soon-Ja| last3 = Kim| first3 = Wan-Kyu| last4 = Yun| first4 = Bong-Sik}}</ref> or ''[[Phellinus]]''.<ref>{{Cite journal | doi=10.1016/j.bmc.2007.03.039 |title=Highly oxygenated and unsaturated metabolites providing a diversity of hispidin class antioxidants in the medicinal mushrooms Inonotus and Phellinus |year=2007 |last1=Lee |first1=In-Kyoung |last2=Yun |first2=Bong-Sik |journal=Bioorganic & Medicinal Chemistry |volume=15 |issue=10 |pages=3309–14 |pmid=17387019}}</ref><ref name="pmid18827365">{{cite journal |doi=10.1248/bpb.31.1968 |title=Protein glycation inhibitors from the fruiting body of ''Phellinus linteus'' |journal=Biological & Pharmaceutical Bulletin |volume=31 |issue=10 |pages=1968–72 |date=October 2008 |pmid=18827365 |url=https://www.jstage.jst.go.jp/article/bpb/31/10/31_10_1968/_article |last1=Lee |first1=Yeon Sil |last2=Kang |first2=Young-Hee |last3=Jung |first3=Ju-Young |last4=Lee |first4=Sanghyun |last5=Ohuchi |first5=Kazuo |last6=Shin |first6=Kuk Hyun |last7=Kang |first7=Il-Jun |last8=Park |first8=Jung Han Yoon |last9=Shin |first9=Hyun-Kyung|last10=Lim |first10=Soon Sung |display-authors=8 |doi-access=free }}</ref> Hispidin is a precursor of [[fungal]] [[luciferin]], a compound responsible for light emission by luminous mushrooms. |
||
== See also == |
== See also == |
||
| Line 53: | Line 51: | ||
{{reflist}} |
{{reflist}} |
||
[[Category: |
[[Category:Hispidins]] |
||
{{ |
{{aromatic-stub}} |
||
Latest revision as of 12:53, 12 December 2021
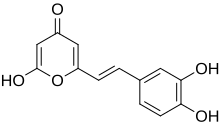
| |
| Names | |
|---|---|
| Preferred IUPAC name
6-[(1E)-2-(3,4-Dihydroxyphenyl)ethen-1-yl]-4-hydroxy-2H-pyran-2-one | |
| Other names
6-(3,4-Dihydroxystyryl)-4-hydroxy-2-pyrone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H10O5 | |
| Molar mass | 246.218 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hispidin is a natural substance. It can also be synthesized.[1]
Hispidin 4-O-β-D-glucopyranoside can be found in Pteris ensiformis[2] whereas hispidin derivatives, such as phellibaumins, can be found in the edible mushroom Inonotus xeranticus[3] or Phellinus.[4][5] Hispidin is a precursor of fungal luciferin, a compound responsible for light emission by luminous mushrooms.
See also
[edit]References
[edit]- ^ Gonindard, C.; Bergonz i, C.; Denier, C.; Sergheraert, C.; Klaebe, A.; Chavant, L.; Hollande, E. (1997). "Synthetic hispidin, a PKC inhibitor, is more cytotoxic toward cancer cells than normal cells in vitro". Cell Biology and Toxicology. 13 (3): 141–53. doi:10.1023/A:1007321227010. PMID 9088624. S2CID 755744.
- ^ Chen, Y.; Chang, F.; Lin, Y.; Wang, L.; Chen, J.; Wu, Y.; Wu, M. (2007). "Identification of phenolic antioxidants from Sword Brake fern (Pteris ensiformis Burm.)". Food Chemistry. 105: 48–56. doi:10.1016/j.foodchem.2007.03.055.
- ^ Lee, In-Kyoung; Seok, Soon-Ja; Kim, Wan-Kyu; Yun, Bong-Sik (2006). "Hispidin Derivatives from the Mushroom Inonotusxeranticusand Their Antioxidant Activity". Journal of Natural Products. 69 (2): 299–301. doi:10.1021/np050453n. PMID 16499338.
- ^ Lee, In-Kyoung; Yun, Bong-Sik (2007). "Highly oxygenated and unsaturated metabolites providing a diversity of hispidin class antioxidants in the medicinal mushrooms Inonotus and Phellinus". Bioorganic & Medicinal Chemistry. 15 (10): 3309–14. doi:10.1016/j.bmc.2007.03.039. PMID 17387019.
- ^ Lee, Yeon Sil; Kang, Young-Hee; Jung, Ju-Young; Lee, Sanghyun; Ohuchi, Kazuo; Shin, Kuk Hyun; Kang, Il-Jun; Park, Jung Han Yoon; et al. (October 2008). "Protein glycation inhibitors from the fruiting body of Phellinus linteus". Biological & Pharmaceutical Bulletin. 31 (10): 1968–72. doi:10.1248/bpb.31.1968. PMID 18827365.
