TC-S 7001: Difference between revisions
Appearance
Content deleted Content added
No edit summary |
Importing Wikidata short description: "Chemical compound" (Shortdesc helper) |
||
| (13 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical compound}} |
|||
{{Drugbox |
{{Drugbox |
||
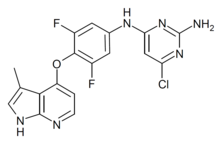
| IUPAC_name = 6-chloro-4-N-[3,5-difluoro-4-[(3-methyl-1H-pyrrolo[2,3-b]pyridin-4-yl)oxy]phenyl]pyrimidine-2,4-diamine |
| IUPAC_name = 6-chloro-4-N-[3,5-difluoro-4-[(3-methyl-1H-pyrrolo[2,3-b]pyridin-4-yl)oxy]phenyl]pyrimidine-2,4-diamine |
||
| Line 6: | Line 7: | ||
| legal_US = |
| legal_US = |
||
| legal_status = |
| legal_status = |
||
<!--Pharmacokinetic data--> |
<!--Pharmacokinetic data--> |
||
| bioavailability = |
| bioavailability = |
||
| Line 12: | Line 12: | ||
| elimination_half-life = |
| elimination_half-life = |
||
| excretion = |
| excretion = |
||
<!--Identifiers--> |
<!--Identifiers--> |
||
| CAS_number = 867017-68-3 |
| CAS_number = 867017-68-3 |
||
| PubChem = 11524200 |
| PubChem = 11524200 |
||
| ChemSpiderID = |
| ChemSpiderID = 9698986 |
||
| ChEMBL = 1971943 |
|||
<!--Chemical data--> |
<!--Chemical data--> |
||
| C=18 | H=13 | Cl=1 | F=2 | N=6 | O=1 |
| C=18 | H=13 | Cl=1 | F=2 | N=6 | O=1 |
||
| Line 25: | Line 24: | ||
}} |
}} |
||
''' |
'''TC-S 7001''' ('''Azaindole-1''') is a drug which acts as a potent and selective inhibitor of the [[enzyme]] [[Rho kinase]], with an IC<sub>50</sub> of 0.6 nM at [[ROCK1]] and 1.1 nM at [[ROCK2]].<ref>{{cite journal | vauthors = Kast R, Schirok H, Figueroa-Pérez S, Mittendorf J, Gnoth MJ, Apeler H, Lenz J, Franz JK, Knorr A, Hütter J, Lobell M, Zimmermann K, Münter K, Augstein KH, Ehmke H, Stasch JP | display-authors = 6 | title = Cardiovascular effects of a novel potent and highly selective azaindole-based inhibitor of Rho-kinase | journal = British Journal of Pharmacology | volume = 152 | issue = 7 | pages = 1070–80 | date = December 2007 | pmid = 17934515 | doi = 10.1038/sj.bjp.0707484 | pmc = 2095102 }}</ref> It has [[vasodilator]]y effects and has been used in research for a variety of applications.<ref>{{cite journal | vauthors = Dahal BK, Kosanovic D, Pamarthi PK, Sydykov A, Lai YJ, Kast R, Schirok H, Stasch JP, Ghofrani HA, Weissmann N, Grimminger F, Seeger W, Schermuly RT | display-authors = 6 | title = Therapeutic efficacy of azaindole-1 in experimental pulmonary hypertension | journal = The European Respiratory Journal | volume = 36 | issue = 4 | pages = 808–18 | date = October 2010 | pmid = 20530035 | doi = 10.1183/09031936.00140309 | s2cid = 10991200 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Pankey EA, Byun RJ, Smith WB, Bhartiya M, Bueno FR, Badejo AM, Stasch JP, Murthy SN, Nossaman BD, Kadowitz PJ | display-authors = 6 | title = The Rho kinase inhibitor azaindole-1 has long-acting vasodilator activity in the pulmonary vascular bed of the intact chest rat | journal = Canadian Journal of Physiology and Pharmacology | volume = 90 | issue = 7 | pages = 825–35 | date = July 2012 | pmid = 22591047 | doi = 10.1139/y2012-061 }}</ref><ref>{{cite journal | vauthors = Lasker GF, Pankey EA, Allain AV, Murthy SN, Stasch JP, Kadowitz PJ | title = The selective Rho-kinase inhibitor azaindole-1 has long-lasting erectile activity in the rat | journal = Urology | volume = 81 | issue = 2 | pages = 465.e7–14 | date = February 2013 | pmid = 23374844 | doi = 10.1016/j.urology.2012.10.039 | pmc = 3564057 }}</ref> |
||
== See also == |
|||
* [[Rho kinase inhibitor]] |
|||
== References == |
== References == |
||
| Line 32: | Line 34: | ||
{{pharm-stub}} |
{{pharm-stub}} |
||
[[Category:Enzyme inhibitors]] |
[[Category:Enzyme inhibitors]] |
||
[[Category:Pyrrolopyridines]] |
|||
[[Category:Pyrimidines]] |
|||
[[Category:Fluoroarenes]] |
|||
[[Category:Chloroarenes]] |
|||
[[Category:Aromatic ethers]] |
|||
Latest revision as of 10:26, 23 April 2022
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H13ClF2N6O |
| Molar mass | 402.79 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
TC-S 7001 (Azaindole-1) is a drug which acts as a potent and selective inhibitor of the enzyme Rho kinase, with an IC50 of 0.6 nM at ROCK1 and 1.1 nM at ROCK2.[1] It has vasodilatory effects and has been used in research for a variety of applications.[2][3][4]
See also
[edit]References
[edit]- ^ Kast R, Schirok H, Figueroa-Pérez S, Mittendorf J, Gnoth MJ, Apeler H, et al. (December 2007). "Cardiovascular effects of a novel potent and highly selective azaindole-based inhibitor of Rho-kinase". British Journal of Pharmacology. 152 (7): 1070–80. doi:10.1038/sj.bjp.0707484. PMC 2095102. PMID 17934515.
- ^ Dahal BK, Kosanovic D, Pamarthi PK, Sydykov A, Lai YJ, Kast R, et al. (October 2010). "Therapeutic efficacy of azaindole-1 in experimental pulmonary hypertension". The European Respiratory Journal. 36 (4): 808–18. doi:10.1183/09031936.00140309. PMID 20530035. S2CID 10991200.
- ^ Pankey EA, Byun RJ, Smith WB, Bhartiya M, Bueno FR, Badejo AM, et al. (July 2012). "The Rho kinase inhibitor azaindole-1 has long-acting vasodilator activity in the pulmonary vascular bed of the intact chest rat". Canadian Journal of Physiology and Pharmacology. 90 (7): 825–35. doi:10.1139/y2012-061. PMID 22591047.
- ^ Lasker GF, Pankey EA, Allain AV, Murthy SN, Stasch JP, Kadowitz PJ (February 2013). "The selective Rho-kinase inhibitor azaindole-1 has long-lasting erectile activity in the rat". Urology. 81 (2): 465.e7–14. doi:10.1016/j.urology.2012.10.039. PMC 3564057. PMID 23374844.
