Ferrier rearrangement: Difference between revisions
m ISBNs (Build KE) |
m →Mechanism: task, replaced: J.Serb.Chem.Soc. → J. Serb. Chem. Soc. |
||
| (18 intermediate revisions by 11 users not shown) | |||
| Line 1: | Line 1: | ||
{{ |
{{distinguish|text=the [[Ferrier carbocyclization]] (also called Ferrier II reaction), a reaction discovered by the same chemist}} |
||
The '''Ferrier rearrangement''' is an [[organic reaction]] that involves a [[nucleophilic substitution]] reaction combined with an [[allylic shift]] in a [[glycal]] (a 2,3-[[unsaturated compound|unsaturated]] [[glycoside]]). It was discovered by the [[carbohydrate]] chemist [[Robert J. Ferrier]].<ref>{{cite journal|last=Ferrier|first=Robert J.|year=1979|title=Unsaturated Carbohydrates. Part 21. A Carboxylic Ring Closure of a Hex-5-enopyranoside Derivative|journal=J. Chem. Soc. |
The '''Ferrier rearrangement''' is an [[organic reaction]] that involves a [[nucleophilic substitution]] reaction combined with an [[allylic shift]] in a [[glycal]] (a 2,3-[[unsaturated compound|unsaturated]] [[glycoside]]). It was discovered by the [[carbohydrate]] chemist [[Robert J. Ferrier]].<ref>{{cite journal|last=Ferrier|first=Robert J.|year=1979|title=Unsaturated Carbohydrates. Part 21. A Carboxylic Ring Closure of a Hex-5-enopyranoside Derivative|journal=J. Chem. Soc. Perkin Trans. 1|pages=1455–1458|doi=10.1039/P19790001455}}</ref><ref>{{cite journal|last1=Ferrier|first1=Robert J.|last2=Zubkov|first2=O. A.|journal=[[Org. React.]]|year=2003|volume=62|doi=10.1002/0471264180.or062.04|title=Transformation of Glycals into 2,3-Unsaturated Glycosyl Derivatives|pages=569–736|isbn=0-471-26418-0}}</ref> |
||
[[File:Ferrier rearrangement.svg|center|800px|A typical Ferrier rearrangement]] |
[[File:Ferrier rearrangement.svg|center|800px|A typical Ferrier rearrangement]] |
||
== Mechanism == |
== Mechanism == |
||
In the first step, a delocalized allyloxocarbenium ion ('''2''') is formed, typically with the aid of a [[Lewis acid]] like [[indium(III) chloride]] or [[boron trifluoride]]. This ion reacts [[in situ#Chemistry and chemical engineering|in situ]] with an alcohol, yielding a mixture of the α ('''3''') and β ('''4''') [[anomer]]s of the 2-glycoside, with the double bond shifted to position 3,4.<ref name="Konstantinović">{{cite journal|last=Konstantinović|first=Stanimir |
In the first step, a delocalized allyloxocarbenium ion ('''2''') is formed, typically with the aid of a [[Lewis acid]] like [[indium(III) chloride]] or [[boron trifluoride]]. This ion reacts [[in situ#Chemistry and chemical engineering|in situ]] with an alcohol, yielding a mixture of the α ('''3''') and β ('''4''') [[anomer]]s of the 2-glycoside, with the double bond shifted to position 3,4.<ref name="Konstantinović">{{cite journal|last=Konstantinović|first=Stanimir|year=2001|title=The Ferrier rearrangement as the key step in the synthesis of C7–C16-alkyl 2,3-dideoxy glucosides from glucose and C7–C16-alkanols|journal=J. Serb. Chem. Soc.|volume=66|issue=8|pages=499–505|doi=10.2298/JSC0108499K|url=http://www.shd.org.rs/JSCS/Vol66/No8/V66-No8-01.pdf|display-authors=etal|doi-access=free}}</ref> |
||
== Examples == |
== Examples == |
||
| Line 18: | Line 18: | ||
| [[methanol]] |
| [[methanol]] |
||
| in [[dichloromethane]] |
| in [[dichloromethane]] |
||
| α:β = 7:1<ref>{{cite journal|last=Boga|first=S. B.| |
| α:β = 7:1<ref>{{cite journal|last=Boga|first=S. B.|author2=Balasubramanian, K. K.|year=2004|title=Indium trichloride catalyzed Ferrier rearrangement – facile synthesis of 2,3-unsaturated glycosides|journal=[[Arkivoc]]|pages=87–102|url=http://www.arkat-usa.org/ark/journal/2004/I08_Narasimhan/1099/1099.asp}} ([[open access (publishing)|open access]] publication)</ref> |
||
|- |
|- |
||
| [[dioxane]] |
| [[dioxane]] |
||
| [[water]] |
| [[water]] |
||
| heating |
| heating |
||
| 75% yield<ref>{{cite journal|journal=J. Am. Chem. Soc.|year=1970|volume=92|pages=5288–5290|doi=10.1021/ja00720a087|title=4,6-Di-O-acetyl-aldehydo-2,3-dideoxy-D-erythro-trans-hex-2-enose. Probable reason for the 'al' in Emil Fischer's triacetyl glucal| |
| 75% yield<ref>{{cite journal|journal=J. Am. Chem. Soc.|year=1970|volume=92|pages=5288–5290|doi=10.1021/ja00720a087|title=4,6-Di-O-acetyl-aldehydo-2,3-dideoxy-D-erythro-trans-hex-2-enose. Probable reason for the 'al' in Emil Fischer's triacetyl glucal|author1=Bert. Fraser- Reid |author2=Bruno. Radatus |issue=17}}</ref> |
||
|- |
|- |
||
| [[Tin(IV) chloride|SnCl<sub>4</sub>]] |
| [[Tin(IV) chloride|SnCl<sub>4</sub>]] |
||
| methanol |
| methanol |
||
| in dichloromethane, –78 °C, 10 min |
| in dichloromethane, –78 °C, 10 min |
||
| 83% yield, α:β = 86:14<ref>{{cite journal|journal=J. Org. Chem.|year=1994|volume=59|pages=2848|doi=10.1021/jo00089a034|title=Simple Designs for the Construction of Complex trans-Fused Polyether Toxin Frameworks. A Linear Strategy Based on Entropically Favored Oxirane Ring Enlargement in Epoxycycloalkenes Followed by Carbon-Carbon or Carbon-Oxygen Bond-Forming Cyclizations| |
| 83% yield, α:β = 86:14<ref>{{cite journal|journal=J. Org. Chem.|year=1994|volume=59|pages=2848|doi=10.1021/jo00089a034|title=Simple Designs for the Construction of Complex trans-Fused Polyether Toxin Frameworks. A Linear Strategy Based on Entropically Favored Oxirane Ring Enlargement in Epoxycycloalkenes Followed by Carbon-Carbon or Carbon-Oxygen Bond-Forming Cyclizations|author1=Eleuterio Alvarez |author2=Maria T. Diaz |author3=Ricardo Perez |author4=Jose L. Ravelo |author5=Alicia Regueiro |author6=Jose A. Vera |author7=Dacil Zurita |author8=Julio D. Martin |issue=10}}</ref> |
||
|- |
|- |
||
| [[boron trifluoride|BF<sub>3</sub>]]·[[diethylether|O(C<sub>2</sub>H<sub>5</sub>)<sub>2</sub>]] |
| [[boron trifluoride|BF<sub>3</sub>]]·[[diethylether|O(C<sub>2</sub>H<sub>5</sub>)<sub>2</sub>]] |
||
| [[isopropanol]] |
| [[isopropanol]] |
||
| in dichloromethane, [[room temperature|RT]], 24 hr |
| in dichloromethane, [[room temperature|RT]], 24 hr |
||
| 95% yield<ref>{{cite journal|journal=Journal of the Chemical Society C Organic|year=1969|pages= |
| 95% yield<ref>{{cite journal|journal=Journal of the Chemical Society C: Organic|year=1969|pages=570–575|doi=10.1039/J39690000570|title=Unsaturated carbohydrates. Part IX. Synthesis of 2,3-dideoxy-α-D-erythro-hex-2-enopyranosides from tri-O-acetyl-D-glucal|author=Ferrier, R. J.|last2=Prasad|first2=N.|issue=4}}</ref><ref>{{cite journal|journal=Journal of the Chemical Society C: Organic|year=1969|pages=575–580|doi=10.1039/J39690000575|title=Unsaturated carbohydrates. Part X. Epoxidations and hydroxylations of 2,3-dideoxy-α-D-hex-2-enopyranosides. The four methyl 4,6-di-O-acetyl-2,3-anhydro-α-D-hexopyranosides|author=Ferrier, R. J.|last2=Prasad|first2=N.|issue=4}}</ref> |
||
|- |
|- |
||
| [[Zinc chloride|ZnCl<sub>2</sub>]] |
| [[Zinc chloride|ZnCl<sub>2</sub>]] |
||
| [[ethanol]] |
| [[ethanol]] |
||
| in [[toluene]], RT, 30–60 min |
| in [[toluene]], RT, 30–60 min |
||
| 65–95% yield, α:β = 89:11<ref>{{cite journal|year=2000|journal=Journal of the Chemical Society Perkin Transactions 1| |
| 65–95% yield, α:β = 89:11<ref>{{cite journal|last2=Picton|first2=Mark R.|year=2000|title=Catalytic tin radical mediated tricyclisations. Part 1. Monocyclisation studies|journal=Journal of the Chemical Society, Perkin Transactions 1|issue=10|pages=1559|doi=10.1039/b000661k|author=Kelly, David R.}}</ref><ref>{{cite journal|last2=Picton|first2=Mark R.|year=2000|title=Catalytic tin radical mediated tricyclisations. Part 2.|journal=Journal of the Chemical Society, Perkin Transactions 1|issue=10|pages=1571|doi=10.1039/b000662i|author=Kelly, David R.}}</ref> |
||
|- |
|- |
||
| BF<sub>3</sub>·O(C<sub>2</sub>H<sub>5</sub>)<sub>2</sub> |
| BF<sub>3</sub>·O(C<sub>2</sub>H<sub>5</sub>)<sub>2</sub> |
||
| [[benzyl alcohol]] |
| [[benzyl alcohol]] |
||
| in dichloromethane, –20 °C to RT, 1 hr |
| in dichloromethane, –20 °C to RT, 1 hr |
||
| 98% yield<ref>{{cite journal|year=1999|journal=Chemical Communications |
| 98% yield<ref>{{cite journal|year=1999|journal=Chemical Communications|pages=1733–1734|doi=10.1039/a904991f|title=Synthesis of amino-sugars using the directed dihydroxylation reaction|author=Donohoe, Timothy J.|last2=Blades|first2=Kevin|last3=Helliwell|first3=Madeleine|issue=17}}</ref> |
||
|} |
|} |
||
== Modifications == |
== Modifications == |
||
=== Forming of C-glycosides === |
=== Forming of C-glycosides === |
||
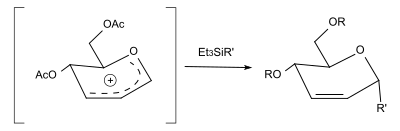
By replacing the alcohol with a [[silane]], C-glycosides can be formed. With [[triethylsilane]] (R'=H), the reaction yields a 2,3-unsaturated |
By replacing the alcohol with a [[silane]], C-glycosides can be formed. With [[triethylsilane]] (R'=H), the reaction yields a 2,3-unsaturated deoxy sugar.<ref name="Konstantinović" /> |
||
[[File:Ferrier rearrangement C-glycoside.svg|400px|none|Forming of a C-glycoside via Ferrier rearrangement]] |
[[File:Ferrier rearrangement C-glycoside.svg|400px|none|Forming of a C-glycoside via Ferrier rearrangement]] |
||
| Line 66: | Line 67: | ||
== References == |
== References == |
||
{{reflist}} |
{{reflist|35em}} |
||
[[Category:Carbohydrate chemistry]] |
[[Category:Carbohydrate chemistry]] |
||
[[Category:Rearrangement reactions]] |
[[Category:Rearrangement reactions]] |
||
[[Category:Name reactions]] |
[[Category:Name reactions]] |
||
[[zh:Ferrier重排反应]] |
|||
Latest revision as of 19:28, 6 July 2022
The Ferrier rearrangement is an organic reaction that involves a nucleophilic substitution reaction combined with an allylic shift in a glycal (a 2,3-unsaturated glycoside). It was discovered by the carbohydrate chemist Robert J. Ferrier.[1][2]
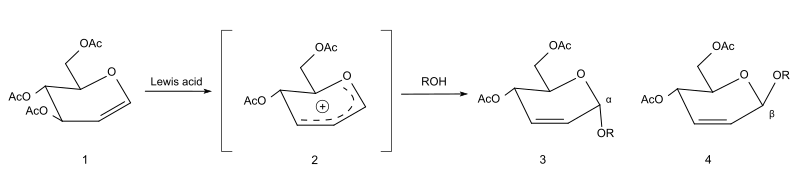
Mechanism
[edit]In the first step, a delocalized allyloxocarbenium ion (2) is formed, typically with the aid of a Lewis acid like indium(III) chloride or boron trifluoride. This ion reacts in situ with an alcohol, yielding a mixture of the α (3) and β (4) anomers of the 2-glycoside, with the double bond shifted to position 3,4.[3]
Examples
[edit]| Lewis acid | Alcohol | Conditions | Results |
|---|---|---|---|
| InCl3 | methanol | in dichloromethane | α:β = 7:1[4] |
| dioxane | water | heating | 75% yield[5] |
| SnCl4 | methanol | in dichloromethane, –78 °C, 10 min | 83% yield, α:β = 86:14[6] |
| BF3·O(C2H5)2 | isopropanol | in dichloromethane, RT, 24 hr | 95% yield[7][8] |
| ZnCl2 | ethanol | in toluene, RT, 30–60 min | 65–95% yield, α:β = 89:11[9][10] |
| BF3·O(C2H5)2 | benzyl alcohol | in dichloromethane, –20 °C to RT, 1 hr | 98% yield[11] |
Modifications
[edit]Forming of C-glycosides
[edit]By replacing the alcohol with a silane, C-glycosides can be formed. With triethylsilane (R'=H), the reaction yields a 2,3-unsaturated deoxy sugar.[3]
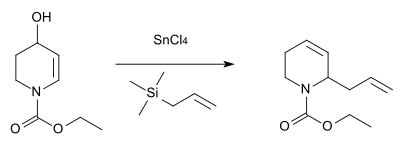
Nitrogen analogue
[edit]An analogous reaction with nitrogen as the heteroatom was described in 1984 for the synthesis of the antibiotic substance streptazolin.[12]
References
[edit]- ^ Ferrier, Robert J. (1979). "Unsaturated Carbohydrates. Part 21. A Carboxylic Ring Closure of a Hex-5-enopyranoside Derivative". J. Chem. Soc. Perkin Trans. 1: 1455–1458. doi:10.1039/P19790001455.
- ^ Ferrier, Robert J.; Zubkov, O. A. (2003). "Transformation of Glycals into 2,3-Unsaturated Glycosyl Derivatives". Org. React. 62: 569–736. doi:10.1002/0471264180.or062.04. ISBN 0-471-26418-0.
- ^ a b Konstantinović, Stanimir; et al. (2001). "The Ferrier rearrangement as the key step in the synthesis of C7–C16-alkyl 2,3-dideoxy glucosides from glucose and C7–C16-alkanols" (PDF). J. Serb. Chem. Soc. 66 (8): 499–505. doi:10.2298/JSC0108499K.
- ^ Boga, S. B.; Balasubramanian, K. K. (2004). "Indium trichloride catalyzed Ferrier rearrangement – facile synthesis of 2,3-unsaturated glycosides". Arkivoc: 87–102. (open access publication)
- ^ Bert. Fraser- Reid; Bruno. Radatus (1970). "4,6-Di-O-acetyl-aldehydo-2,3-dideoxy-D-erythro-trans-hex-2-enose. Probable reason for the 'al' in Emil Fischer's triacetyl glucal". J. Am. Chem. Soc. 92 (17): 5288–5290. doi:10.1021/ja00720a087.
- ^ Eleuterio Alvarez; Maria T. Diaz; Ricardo Perez; Jose L. Ravelo; Alicia Regueiro; Jose A. Vera; Dacil Zurita; Julio D. Martin (1994). "Simple Designs for the Construction of Complex trans-Fused Polyether Toxin Frameworks. A Linear Strategy Based on Entropically Favored Oxirane Ring Enlargement in Epoxycycloalkenes Followed by Carbon-Carbon or Carbon-Oxygen Bond-Forming Cyclizations". J. Org. Chem. 59 (10): 2848. doi:10.1021/jo00089a034.
- ^ Ferrier, R. J.; Prasad, N. (1969). "Unsaturated carbohydrates. Part IX. Synthesis of 2,3-dideoxy-α-D-erythro-hex-2-enopyranosides from tri-O-acetyl-D-glucal". Journal of the Chemical Society C: Organic (4): 570–575. doi:10.1039/J39690000570.
- ^ Ferrier, R. J.; Prasad, N. (1969). "Unsaturated carbohydrates. Part X. Epoxidations and hydroxylations of 2,3-dideoxy-α-D-hex-2-enopyranosides. The four methyl 4,6-di-O-acetyl-2,3-anhydro-α-D-hexopyranosides". Journal of the Chemical Society C: Organic (4): 575–580. doi:10.1039/J39690000575.
- ^ Kelly, David R.; Picton, Mark R. (2000). "Catalytic tin radical mediated tricyclisations. Part 1. Monocyclisation studies". Journal of the Chemical Society, Perkin Transactions 1 (10): 1559. doi:10.1039/b000661k.
- ^ Kelly, David R.; Picton, Mark R. (2000). "Catalytic tin radical mediated tricyclisations. Part 2". Journal of the Chemical Society, Perkin Transactions 1 (10): 1571. doi:10.1039/b000662i.
- ^ Donohoe, Timothy J.; Blades, Kevin; Helliwell, Madeleine (1999). "Synthesis of amino-sugars using the directed dihydroxylation reaction". Chemical Communications (17): 1733–1734. doi:10.1039/a904991f.
- ^
Kozikowski, AP, Pyeong-uk Park (1984). "Synthesis of 2-substituted .DELTA.3-piperidines: the nitrogen analog of the Ferrier rearrangement. An approach to streptazolin". J. Org. Chem. 49 (9): 1674–1676. doi:10.1021/jo00183a044.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
