Antimony(III) acetate: Difference between revisions
Appearance
Content deleted Content added
Thejoester69 (talk | contribs) No edit summary |
|||
| (44 intermediate revisions by 28 users not shown) | |||
| Line 1: | Line 1: | ||
{{chembox |
|||
Dear Pathetic wikipedia user, |
|||
| Verifiedfields = changed |
|||
I have changed this article in the liberation of Freedom! Why? because if your looking up this seemingly obscure article in search of knowledge about the precious "Antimony(III)acetate I have found it before you and changed it. This is for your own good. There is no one in their right mind who would need this article and if their was I hopefully saved their sanity. |
|||
| Watchedfields = changed |
|||
| verifiedrevid = 429853293 |
|||
| Name = Antimony(III) acetate |
|||
| ImageFile = Antimony(III)-acetate-xtal-1980-CM-3D-balls.png |
|||
| ImageSize = 250px |
|||
| ImageName = Antimony(III) acetate |
|||
| IUPACName = Antimony(III) acetate |
|||
| OtherNames = Antimony triacetate<br />Acetic acid, antimony(3+) salt |
|||
|Section1={{Chembox Identifiers |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| ChemSpiderID = 21839 |
|||
| InChI = 1/3C2H4O2.Sb.3H/c3*1-2(3)4;;;;/h3*1H3,(H,3,4);;;;/q;;;+3;;;/p-3/r3C2H4O2.H3Sb/c3*1-2(3)4;/h3*1H3,(H,3,4);1H3/q;;;+3/p-3 |
|||
| InChIKey = NSMVVPJZMRQLMR-ZHOQVWKLAW |
|||
| InChI1 = 1/3C2H4O2.Sb/c3*1-2(3)4;/h3*1H3,(H,3,4);/q;;;+3/p-3 |
|||
| InChIKey1 = JVLRYPRBKSMEBF-DFZHHIFOAU |
|||
| SMILES = O=C(C)O[Sb](OC(C)=O)OC(C)=O |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChI = 1S/3C2H4O2.Sb/c3*1-2(3)4;/h3*1H3,(H,3,4);/q;;;+3/p-3 |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = JVLRYPRBKSMEBF-UHFFFAOYSA-K |
|||
| CASNo_Ref = {{cascite|correct|??}} |
|||
| CASNo = 6923-52-0 |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = F6Z6EKG4QY |
|||
| PubChem = 16685080 |
|||
| RTECS = AF4200000 |
|||
}} |
|||
|Section2={{Chembox Properties |
|||
| Formula = Sb(CH<sub>3</sub>COO)<sub>3</sub> |
|||
| Appearance = White powder |
|||
| Density = 1.22 g/cm<sup>3</sup> (20 °C) |
|||
| Solubility = |
|||
| MeltingPtC = 128.5 |
|||
| MeltingPt_notes = (decomposes to [[antimony(III) oxide|Sb<sub>2</sub>O<sub>3</sub>]]) |
|||
| BoilingPt = |
|||
| pKa = |
|||
| pKb = |
|||
| Viscosity = |
|||
}} |
|||
|Section3={{Chembox Structure |
|||
| Coordination = |
|||
| CrystalStruct = |
|||
| Dipole = |
|||
}} |
|||
|Section7={{Chembox Hazards |
|||
| MainHazards = |
|||
| NFPA-H = 1 |
|||
| NFPA-F = 0 |
|||
| NFPA-R = 0 |
|||
| LD50 = 4480 mg/kg (rat) |
|||
| REL = TWA 0.5 mg/m<sup>3</sup> (as Sb)<ref name=PGCH>{{PGCH|0036}}</ref> |
|||
| PEL = TWA 0.5 mg/m<sup>3</sup> (as Sb)<ref name=PGCH/> |
|||
}} |
|||
}} |
|||
'''Antimony(III) acetate''' is the compound of [[antimony]] with the [[chemical formula]] of Sb(CH<sub>3</sub>CO<sub>2</sub>)<sub>3</sub>. It is a white powder, is moderately water-soluble, and is used as a [[catalyst]] in the production of [[polyester]]s. |
|||
Sincerely the Sinful Sixty-niner |
|||
==Preparation== |
|||
P.S. What is worse than a worm in your apple?... your whole family being murdered. |
|||
It can be prepared by the reaction of [[antimony(III) oxide]] with [[acetic anhydride]]: |
|||
:Sb<sub>2</sub>O<sub>3</sub> + 3 C<sub>4</sub>H<sub>6</sub>O<sub>3</sub> → 2 Sb(CH<sub>3</sub>CO<sub>2</sub>)<sub>3</sub> |
|||
==Structure== |
|||
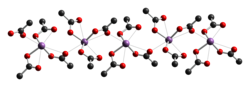
The crystal structure of antimony(III) acetate has been determined by [[X-ray crystallography]]. It consists of discrete Sb(OAc)<sub>3</sub> monomers with monodentate [[acetate]] ligands. The monomers are linked together into chains by weaker C=O···Sb [[intermolecular interaction]]s.<ref>{{ cite journal | journal = [[Dalton Transactions|J. Chem. Soc., Dalton Trans.]] | year = 1980 | pages = 1292–1296 | first1 = M. | last1 = Hall | first2 = D. B. | last2 = Sowerby | title = Antimony(III) acetate and thioacetate: spectra and crystal structures | doi = 10.1039/DT9800001292 | issue = 8 }}</ref> |
|||
==References== |
|||
{{reflist}} |
|||
{{Inorganic-compound-stub}} |
|||
{{Antimony compounds}} |
|||
{{clear}} |
|||
{{Acetates}} |
|||
[[Category:Antimony(III) compounds]] |
|||
[[Category:Acetates]] |
|||
Latest revision as of 07:22, 29 August 2022
| |
| Names | |
|---|---|
| IUPAC name
Antimony(III) acetate
| |
| Other names
Antimony triacetate
Acetic acid, antimony(3+) salt | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.027.312 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Sb(CH3COO)3 | |
| Appearance | White powder |
| Density | 1.22 g/cm3 (20 °C) |
| Melting point | 128.5 °C (263.3 °F; 401.6 K) (decomposes to Sb2O3) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
4480 mg/kg (rat) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.5 mg/m3 (as Sb)[1] |
REL (Recommended)
|
TWA 0.5 mg/m3 (as Sb)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Antimony(III) acetate is the compound of antimony with the chemical formula of Sb(CH3CO2)3. It is a white powder, is moderately water-soluble, and is used as a catalyst in the production of polyesters.
Preparation
[edit]It can be prepared by the reaction of antimony(III) oxide with acetic anhydride:
- Sb2O3 + 3 C4H6O3 → 2 Sb(CH3CO2)3
Structure
[edit]The crystal structure of antimony(III) acetate has been determined by X-ray crystallography. It consists of discrete Sb(OAc)3 monomers with monodentate acetate ligands. The monomers are linked together into chains by weaker C=O···Sb intermolecular interactions.[2]
References
[edit]- ^ a b NIOSH Pocket Guide to Chemical Hazards. "#0036". National Institute for Occupational Safety and Health (NIOSH).
- ^ Hall, M.; Sowerby, D. B. (1980). "Antimony(III) acetate and thioacetate: spectra and crystal structures". J. Chem. Soc., Dalton Trans. (8): 1292–1296. doi:10.1039/DT9800001292.

