Antimony(III) acetate: Difference between revisions
Appearance
Content deleted Content added
m check and adjust {Chembox} temperatures (fmt references, notes, brackets, numerics) using AWB |
|||
| (11 intermediate revisions by 10 users not shown) | |||
| Line 1: | Line 1: | ||
{{chembox |
{{chembox |
||
| Verifiedfields = changed |
|||
| Watchedfields = changed |
| Watchedfields = changed |
||
| verifiedrevid = 429853293 |
| verifiedrevid = 429853293 |
||
| |
| Name = Antimony(III) acetate |
||
| |
| ImageFile = Antimony(III)-acetate-xtal-1980-CM-3D-balls.png |
||
| |
| ImageSize = 250px |
||
| |
| ImageName = Antimony(III) acetate |
||
| |
| IUPACName = Antimony(III) acetate |
||
| |
| OtherNames = Antimony triacetate<br />Acetic acid, antimony(3+) salt |
||
| |
|Section1={{Chembox Identifiers |
||
| ⚫ | |||
| SMILES = CC(=O)[O-].CC(=O)[O-].CC(=O)[O-].[SbH3+3] |
|||
| ⚫ | |||
| ChemSpiderID = 21839 |
| ChemSpiderID = 21839 |
||
| InChI = 1/3C2H4O2.Sb.3H/c3*1-2(3)4;;;;/h3*1H3,(H,3,4);;;;/q;;;+3;;;/p-3/r3C2H4O2.H3Sb/c3*1-2(3)4;/h3*1H3,(H,3,4);1H3/q;;;+3/p-3 |
| InChI = 1/3C2H4O2.Sb.3H/c3*1-2(3)4;;;;/h3*1H3,(H,3,4);;;;/q;;;+3;;;/p-3/r3C2H4O2.H3Sb/c3*1-2(3)4;/h3*1H3,(H,3,4);1H3/q;;;+3/p-3 |
||
| Line 16: | Line 16: | ||
| InChI1 = 1/3C2H4O2.Sb/c3*1-2(3)4;/h3*1H3,(H,3,4);/q;;;+3/p-3 |
| InChI1 = 1/3C2H4O2.Sb/c3*1-2(3)4;/h3*1H3,(H,3,4);/q;;;+3/p-3 |
||
| InChIKey1 = JVLRYPRBKSMEBF-DFZHHIFOAU |
| InChIKey1 = JVLRYPRBKSMEBF-DFZHHIFOAU |
||
| |
| SMILES = O=C(C)O[Sb](OC(C)=O)OC(C)=O |
||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChI = 1S/3C2H4O2.Sb/c3*1-2(3)4;/h3*1H3,(H,3,4);/q;;;+3/p-3 |
| StdInChI = 1S/3C2H4O2.Sb/c3*1-2(3)4;/h3*1H3,(H,3,4);/q;;;+3/p-3 |
||
| Line 23: | Line 23: | ||
| CASNo_Ref = {{cascite|correct|??}} |
| CASNo_Ref = {{cascite|correct|??}} |
||
| CASNo = 6923-52-0 |
| CASNo = 6923-52-0 |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = F6Z6EKG4QY |
|||
| PubChem = 16685080 |
| PubChem = 16685080 |
||
| RTECS = AF4200000 |
| RTECS = AF4200000 |
||
}} |
}} |
||
| |
|Section2={{Chembox Properties |
||
| Formula = Sb(CH<sub>3</sub>COO)<sub>3</sub> |
|||
| C = 6 | H = 9 | O = 6 | Sb = 1 |
|||
| |
| Appearance = White powder |
||
| |
| Density = 1.22 g/cm<sup>3</sup> (20 °C) |
||
| |
| Solubility = |
||
| |
| MeltingPtC = 128.5 |
||
| |
| MeltingPt_notes = (decomposes to [[antimony(III) oxide|Sb<sub>2</sub>O<sub>3</sub>]]) |
||
| |
| BoilingPt = |
||
| |
| pKa = |
||
| |
| pKb = |
||
| |
| Viscosity = |
||
}} |
}} |
||
| |
|Section3={{Chembox Structure |
||
| |
| Coordination = |
||
| |
| CrystalStruct = |
||
| |
| Dipole = |
||
}} |
}} |
||
| |
|Section7={{Chembox Hazards |
||
| |
| MainHazards = |
||
| |
| NFPA-H = 1 |
||
| |
| NFPA-F = 0 |
||
| |
| NFPA-R = 0 |
||
| |
| LD50 = 4480 mg/kg (rat) |
||
| REL = TWA 0.5 mg/m<sup>3</sup> (as Sb)<ref name=PGCH>{{PGCH|0036}}</ref> |
|||
| PEL = TWA 0.5 mg/m<sup>3</sup> (as Sb)<ref name=PGCH/> |
|||
}} |
}} |
||
}} |
}} |
||
'''Antimony(III) acetate''' is the |
'''Antimony(III) acetate''' is the compound of [[antimony]] with the [[chemical formula]] of Sb(CH<sub>3</sub>CO<sub>2</sub>)<sub>3</sub>. It is a white powder, is moderately water-soluble, and is used as a [[catalyst]] in the production of [[polyester]]s. |
||
==Preparation== |
==Preparation== |
||
It can be prepared by the reaction of [[antimony(III) oxide]] with [[acetic |
It can be prepared by the reaction of [[antimony(III) oxide]] with [[acetic anhydride]]: |
||
:Sb<sub>2</sub>O<sub>3</sub> + |
:Sb<sub>2</sub>O<sub>3</sub> + 3 C<sub>4</sub>H<sub>6</sub>O<sub>3</sub> → 2 Sb(CH<sub>3</sub>CO<sub>2</sub>)<sub>3</sub> |
||
== |
==Structure== |
||
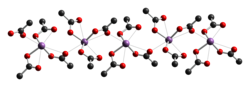
The crystal structure of antimony(III) acetate has been determined by [[X-ray crystallography]]. It consists of discrete Sb(OAc)<sub>3</sub> monomers with monodentate [[acetate]] ligands. The monomers are linked together into chains by weaker C=O···Sb [[intermolecular interaction]]s.<ref>{{ cite journal | journal = [[Dalton Transactions|J. Chem. Soc., Dalton Trans.]] | year = 1980 | pages = 1292–1296 | first1 = M. | last1 = Hall | first2 = D. B. | last2 = Sowerby | title = Antimony(III) acetate and thioacetate: spectra and crystal structures | doi = 10.1039/DT9800001292 | issue = 8 }}</ref> |
The crystal structure of antimony(III) acetate has been determined by [[X-ray crystallography]]. It consists of discrete Sb(OAc)<sub>3</sub> monomers with monodentate [[acetate]] ligands. The monomers are linked together into chains by weaker C=O···Sb [[intermolecular interaction]]s.<ref>{{ cite journal | journal = [[Dalton Transactions|J. Chem. Soc., Dalton Trans.]] | year = 1980 | pages = 1292–1296 | first1 = M. | last1 = Hall | first2 = D. B. | last2 = Sowerby | title = Antimony(III) acetate and thioacetate: spectra and crystal structures | doi = 10.1039/DT9800001292 | issue = 8 }}</ref> |
||
[[File:Antimony(III)-acetate-xtal-1980-CM-3D-balls.png|400px]] |
|||
==References== |
==References== |
||
| Line 70: | Line 72: | ||
{{Antimony compounds}} |
{{Antimony compounds}} |
||
{{clear}} |
|||
{{Acetates}} |
|||
[[Category:Antimony compounds]] |
[[Category:Antimony(III) compounds]] |
||
[[Category:Acetates]] |
[[Category:Acetates]] |
||
Latest revision as of 07:22, 29 August 2022
| |
| Names | |
|---|---|
| IUPAC name
Antimony(III) acetate
| |
| Other names
Antimony triacetate
Acetic acid, antimony(3+) salt | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.027.312 |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Sb(CH3COO)3 | |
| Appearance | White powder |
| Density | 1.22 g/cm3 (20 °C) |
| Melting point | 128.5 °C (263.3 °F; 401.6 K) (decomposes to Sb2O3) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
4480 mg/kg (rat) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.5 mg/m3 (as Sb)[1] |
REL (Recommended)
|
TWA 0.5 mg/m3 (as Sb)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Antimony(III) acetate is the compound of antimony with the chemical formula of Sb(CH3CO2)3. It is a white powder, is moderately water-soluble, and is used as a catalyst in the production of polyesters.
Preparation
[edit]It can be prepared by the reaction of antimony(III) oxide with acetic anhydride:
- Sb2O3 + 3 C4H6O3 → 2 Sb(CH3CO2)3
Structure
[edit]The crystal structure of antimony(III) acetate has been determined by X-ray crystallography. It consists of discrete Sb(OAc)3 monomers with monodentate acetate ligands. The monomers are linked together into chains by weaker C=O···Sb intermolecular interactions.[2]
References
[edit]- ^ a b NIOSH Pocket Guide to Chemical Hazards. "#0036". National Institute for Occupational Safety and Health (NIOSH).
- ^ Hall, M.; Sowerby, D. B. (1980). "Antimony(III) acetate and thioacetate: spectra and crystal structures". J. Chem. Soc., Dalton Trans. (8): 1292–1296. doi:10.1039/DT9800001292.

