Pyrosilicic acid: Difference between revisions
Appearance
Content deleted Content added
Jorge Stolfi (talk | contribs) Created article from some refs |
chem formatting, short description, link Tags: Mobile edit Mobile app edit iOS app edit |
||
| (21 intermediate revisions by 14 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Silicic acid with the formula (HO)3–Si–O–Si–(OH)3}} |
|||
{{Chembox |
{{Chembox |
||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Watchedfields = changed |
| Watchedfields = changed |
||
| verifiedrevid = |
| verifiedrevid = |
||
| IUPACName = Trihydroxysilyl trihydrogen orthosilicate |
| IUPACName = Trihydroxysilyl trihydrogen orthosilicate |
||
| OtherNames = |
| OtherNames = disilicic acid |
||
| ImageFile = |
| ImageFile = Pyrosilicic-acid-3D-balls.png |
||
| ImageSize = |
| ImageSize = 200px |
||
| ImageName = |
| ImageName = Pyrosilicic acid |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| |
| CASNo = 20638-18-0 |
||
| InChIKey1 = |
|||
| CASNo = |
|||
| CASNo_Ref = {{cascite|changed|CAS}} |
| CASNo_Ref = {{cascite|changed|CAS}} |
||
| ⚫ | |||
| UNII_Ref = {{fdacite|changed|FDA}} |
|||
| |
| ChemSpiderID = 55684 |
||
| ⚫ | |||
| ChemSpiderID = |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| |
| ChEBI = 29292 |
||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
| ChEBI_Ref = {{ebicite|correct|EBI}} |
||
| |
| Gmelin = 26937 |
||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChI = 1S/H6O7Si2/c1-8(2,3)7-9(4,5)6/h1-6H |
| StdInChI = 1S/H6O7Si2/c1-8(2,3)7-9(4,5)6/h1-6H |
||
| Line 26: | Line 23: | ||
| StdInChIKey = KDJOAYSYCXTQGG-UHFFFAOYSA-N |
| StdInChIKey = KDJOAYSYCXTQGG-UHFFFAOYSA-N |
||
| SMILES = O[Si](O)(O)O[Si](O)(O)O |
| SMILES = O[Si](O)(O)O[Si](O)(O)O |
||
| InChI = |
|||
| InChIKey = |
|||
| Gmelin = |
|||
}} |
}} |
||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| H=6 | O=7 | Si=2 |
| H=6 | O=7 | Si=2 |
||
| pKa = |
| pKa = |
||
| ConjugateBase = [[Pyrosilicate]] |
|||
| MagSus = |
| MagSus = |
||
}} |
}} |
||
| Line 38: | Line 33: | ||
}} |
}} |
||
'''Pyrosilicic acid''' is the [[chemical compound]] with formula {{ |
'''Pyrosilicic acid''' is the [[chemical compound]] with formula {{chem2|H6Si2O7}} or {{chem2|(HO)3SiOSi(OH)3}}. It is one of the [[silicic acid]]s and has [[pyrosilicate]] as its [[conjugate base]]. |
||
It was synthesized, using nonaqueous solutions, in 2017.<ref name=synthesis>{{cite journal |last1=Igarashi |first1=Masayasu |last2=Matsumoto |first2=Tomohiro |last3=Yagahashi |first3=Fujio |last4=Yamashita |first4=Hiroshi |last5=Ohhara |first5=Takashi |last6=Hanashima |first6=Takayasu |last7=Nakao |first7=Akiko |last8=Moyosh |first8=Taketo |last9=Sato |first9=Kazuhiko |last10=Shimada |first10=Shigeru |title=Non-aqueous selective synthesis of orthosilicic acid and its oligomers |journal=Nature Communications |date=2017 |volume=8 |issue=1 |pages=140 |doi=10.1038/s41467-017-00168-5|pmid=28747652|pmc=5529440|bibcode=2017NatCo...8..140I }}</ref> |
|||
The salts of pyrosilicic acid are called [[pyrosilicate]]s, such as [[sodium pyrosilicate]], and are stable. They occur in nature as a class of [[silicate minerals]], specificlaly the [[sorosilicate]]s. |
|||
:<chem>H6Si2O7(aq) -> 2 SiO2(s) + 3 H2O</chem> |
|||
| ⚫ | Pyrosilicic acid may be present in [[sea water]] and other natural waters at very low concentration.<ref name=goto>Katsumi Goto (1956): "Effect of pH on Polymerization of Silicic Acid". ''Journal of Physical Chemistry'', volume 60, issue 7, pages 1007–1008. {{doi|10.1021/j150541a046}}</ref><ref name=bech>M. F. Bechtold (1955): "Polymerization and Properties of Dilute Aqueous Silicic Acid from Cation Exchange". ''Journal of Physical Chemistry'', volume 59, issue 6, pages 532–541. {{doi|10.1021/j150528a013}}</ref> Compounds formally derived from it, such as [[sodium pyrosilicate]], are found in the [[sorosilicate]] minerals. |
||
==Reactions== |
|||
| ⚫ | |||
==References== |
==References== |
||
{{reflist}} |
{{reflist}} |
||
[[Category:Oxoacids]] |
|||
[[Category:Silicon compounds]] |
[[Category:Silicon compounds]] |
||
Latest revision as of 06:18, 24 September 2022
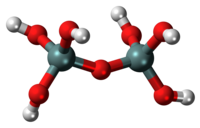
| |
| Names | |
|---|---|
| IUPAC name
Trihydroxysilyl trihydrogen orthosilicate
| |
| Other names
disilicic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| 26937 | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H6O7Si2 | |
| Molar mass | 174.211 g·mol−1 |
| Conjugate base | Pyrosilicate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pyrosilicic acid is the chemical compound with formula H6Si2O7 or (HO)3SiOSi(OH)3. It is one of the silicic acids and has pyrosilicate as its conjugate base.
It was synthesized, using nonaqueous solutions, in 2017.[1]
Pyrosilicic acid may be present in sea water and other natural waters at very low concentration.[2][3] Compounds formally derived from it, such as sodium pyrosilicate, are found in the sorosilicate minerals.
References
[edit]- ^ Igarashi, Masayasu; Matsumoto, Tomohiro; Yagahashi, Fujio; Yamashita, Hiroshi; Ohhara, Takashi; Hanashima, Takayasu; Nakao, Akiko; Moyosh, Taketo; Sato, Kazuhiko; Shimada, Shigeru (2017). "Non-aqueous selective synthesis of orthosilicic acid and its oligomers". Nature Communications. 8 (1): 140. Bibcode:2017NatCo...8..140I. doi:10.1038/s41467-017-00168-5. PMC 5529440. PMID 28747652.
- ^ Katsumi Goto (1956): "Effect of pH on Polymerization of Silicic Acid". Journal of Physical Chemistry, volume 60, issue 7, pages 1007–1008. doi:10.1021/j150541a046
- ^ M. F. Bechtold (1955): "Polymerization and Properties of Dilute Aqueous Silicic Acid from Cation Exchange". Journal of Physical Chemistry, volume 59, issue 6, pages 532–541. doi:10.1021/j150528a013

