Pyrosilicic acid: Difference between revisions
Appearance
Content deleted Content added
Jorge Stolfi (talk | contribs) Relation to disilicic acid. Preparation. |
chem formatting, short description, link Tags: Mobile edit Mobile app edit iOS app edit |
||
| (18 intermediate revisions by 13 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Silicic acid with the formula (HO)3–Si–O–Si–(OH)3}} |
|||
{{Chembox |
{{Chembox |
||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Watchedfields = changed |
| Watchedfields = changed |
||
| verifiedrevid = |
| verifiedrevid = |
||
| IUPACName = Trihydroxysilyl trihydrogen orthosilicate |
| IUPACName = Trihydroxysilyl trihydrogen orthosilicate |
||
| OtherNames = |
| OtherNames = disilicic acid |
||
| ImageFile = Pyrosilicic-acid-3D-balls.png |
| ImageFile = Pyrosilicic-acid-3D-balls.png |
||
| ImageSize = 200px |
| ImageSize = 200px |
||
| ImageName = Pyrosilicic acid |
| ImageName = Pyrosilicic acid |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| |
| CASNo = 20638-18-0 |
||
| InChIKey1 = |
|||
| CASNo = |
|||
| CASNo_Ref = {{cascite|changed|CAS}} |
| CASNo_Ref = {{cascite|changed|CAS}} |
||
| ⚫ | |||
| UNII_Ref = {{fdacite|changed|FDA}} |
|||
| UNII = |
|||
| ⚫ | |||
| ChemSpiderID = 55684 |
| ChemSpiderID = 55684 |
||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| |
| ChEBI = 29292 |
||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
| ChEBI_Ref = {{ebicite|correct|EBI}} |
||
| |
| Gmelin = 26937 |
||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChI = 1S/H6O7Si2/c1-8(2,3)7-9(4,5)6/h1-6H |
| StdInChI = 1S/H6O7Si2/c1-8(2,3)7-9(4,5)6/h1-6H |
||
| Line 26: | Line 23: | ||
| StdInChIKey = KDJOAYSYCXTQGG-UHFFFAOYSA-N |
| StdInChIKey = KDJOAYSYCXTQGG-UHFFFAOYSA-N |
||
| SMILES = O[Si](O)(O)O[Si](O)(O)O |
| SMILES = O[Si](O)(O)O[Si](O)(O)O |
||
| InChI = |
|||
| InChIKey = |
|||
| Gmelin = |
|||
}} |
}} |
||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| H=6 | O=7 | Si=2 |
| H=6 | O=7 | Si=2 |
||
| pKa = |
| pKa = |
||
| ConjugateBase = [[Pyrosilicate]] |
|||
| MagSus = |
| MagSus = |
||
}} |
}} |
||
| Line 38: | Line 33: | ||
}} |
}} |
||
'''Pyrosilicic acid''' is the [[chemical compound]] with formula {{ |
'''Pyrosilicic acid''' is the [[chemical compound]] with formula {{chem2|H6Si2O7}} or {{chem2|(HO)3SiOSi(OH)3}}. It is one of the [[silicic acid]]s and has [[pyrosilicate]] as its [[conjugate base]]. |
||
It was synthesized, using nonaqueous solutions, in 2017.<ref name=synthesis>{{cite journal |last1=Igarashi |first1=Masayasu |last2=Matsumoto |first2=Tomohiro |last3=Yagahashi |first3=Fujio |last4=Yamashita |first4=Hiroshi |last5=Ohhara |first5=Takashi |last6=Hanashima |first6=Takayasu |last7=Nakao |first7=Akiko |last8=Moyosh |first8=Taketo |last9=Sato |first9=Kazuhiko |last10=Shimada |first10=Shigeru |title=Non-aqueous selective synthesis of orthosilicic acid and its oligomers |journal=Nature Communications |date=2017 |volume=8 |issue=1 |pages=140 |doi=10.1038/s41467-017-00168-5|pmid=28747652|pmc=5529440|bibcode=2017NatCo...8..140I }}</ref> |
|||
The salts of pyrosilicic acid are called [[pyrosilicate]]s, such as [[sodium pyrosilicate]], and are stable. They occur in nature as a class of [[silicate minerals]], specificlaly the [[sorosilicate]]s. |
|||
:<chem>H6Si2O7(aq) -> 2 SiO2(s) + 3 H2O</chem> |
|||
| ⚫ | Pyrosilicic acid may be present in [[sea water]] and other natural waters at very low concentration.<ref name=goto>Katsumi Goto (1956): "Effect of pH on Polymerization of Silicic Acid". ''Journal of Physical Chemistry'', volume 60, issue 7, pages 1007–1008. {{doi|10.1021/j150541a046}}</ref><ref name=bech>M. F. Bechtold (1955): "Polymerization and Properties of Dilute Aqueous Silicic Acid from Cation Exchange". ''Journal of Physical Chemistry'', volume 59, issue 6, pages 532–541. {{doi|10.1021/j150528a013}}</ref> Compounds formally derived from it, such as [[sodium pyrosilicate]], are found in the [[sorosilicate]] minerals. |
||
==Reactions== |
|||
| ⚫ | |||
: {{chem|H|2|Si|2|O|5}} (disilicic acid) + 2{{chem|H|2|O}} ↔ {{chem|(HO)|3|Si}}–O–{{chem|Si|(OH)|3}} (pyrosilicic acid) |
|||
It can also be [[hydrolized]] to [[monomer]]ic orthosilicic acid: |
|||
: {{chem|(HO)|3|Si}}–O–{{chem|Si|(OH)|3}} (pyrosilicic acid) + {{chem|H|2|O}} ↔ {{chem|(HO)|3|Si}}–OH + HO–{{chem|Si|(OH)|3}} (orthosilicic acid) |
|||
==Preparation== |
|||
Dilute solutions of pyrosilicic acid can be prepared by removing the [[sodium]] cations from solutions of [[sodium pyrosilicate]] with an [[ion exchange resin]].<ref name=gye>W. E. Gye and W. J. Purdy (1922): "The Poisonous Properties of Colloidal Silica. I: The Effects of the Parenteral Administration of Large Doses" ''British Journal of Experimental Pathology'', volume 3, issue 2, pages 75–85. PMCID: PMC2047780</ref> |
|||
Pyrosilicic acid can be prepared also by dissolving a solution of [[sodium pyrosilicate]], which is naturally alkaline, in a solution of [[sulfuric acid]], so as to rapidly achieve an acidic solution with low silica concentration, such as 0.1% {{chem|Si|O|2}} and pH of 2.5. In those conditions, the polymerization and depolymerization reactions above are fairly slow, and the pyrosilicate or disilicate from the original solution is converted to pyrosilicic acid, which persists for 10 minutes or more.<ref name=GBAlex>G. B. Alexander (1953): "The Reaction of Low Molecular Weight Silicic Acids with Molybdic Acid". ''Journal of the American Chemical Society, volume 75, issue 22, pages 5655–5657. {{doi|10.1021/ja01118a054}}</ref> |
|||
Pyrosilicic acid can also be obtained by controlled hydrolysis of the corresponding organic ester, such as [[hexaethyl pyrosilicate]], with dilute sulfuric acid.<ref name=GBAlex/> |
|||
==References== |
==References== |
||
{{reflist}} |
{{reflist}} |
||
[[Category:Oxoacids]] |
|||
[[Category:Silicon compounds]] |
[[Category:Silicon compounds]] |
||
Latest revision as of 06:18, 24 September 2022
| |
| Names | |
|---|---|
| IUPAC name
Trihydroxysilyl trihydrogen orthosilicate
| |
| Other names
disilicic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| 26937 | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| H6O7Si2 | |
| Molar mass | 174.211 g·mol−1 |
| Conjugate base | Pyrosilicate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
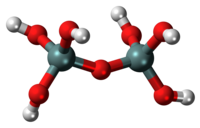
Pyrosilicic acid is the chemical compound with formula H6Si2O7 or (HO)3SiOSi(OH)3. It is one of the silicic acids and has pyrosilicate as its conjugate base.
It was synthesized, using nonaqueous solutions, in 2017.[1]
Pyrosilicic acid may be present in sea water and other natural waters at very low concentration.[2][3] Compounds formally derived from it, such as sodium pyrosilicate, are found in the sorosilicate minerals.
References
[edit]- ^ Igarashi, Masayasu; Matsumoto, Tomohiro; Yagahashi, Fujio; Yamashita, Hiroshi; Ohhara, Takashi; Hanashima, Takayasu; Nakao, Akiko; Moyosh, Taketo; Sato, Kazuhiko; Shimada, Shigeru (2017). "Non-aqueous selective synthesis of orthosilicic acid and its oligomers". Nature Communications. 8 (1): 140. Bibcode:2017NatCo...8..140I. doi:10.1038/s41467-017-00168-5. PMC 5529440. PMID 28747652.
- ^ Katsumi Goto (1956): "Effect of pH on Polymerization of Silicic Acid". Journal of Physical Chemistry, volume 60, issue 7, pages 1007–1008. doi:10.1021/j150541a046
- ^ M. F. Bechtold (1955): "Polymerization and Properties of Dilute Aqueous Silicic Acid from Cation Exchange". Journal of Physical Chemistry, volume 59, issue 6, pages 532–541. doi:10.1021/j150528a013

