Denaverine: Difference between revisions
Appearance
Content deleted Content added
حسن علي البط (talk | contribs) added Category:Ethers using HotCat |
Citation bot (talk | contribs) Add: year. | Use this bot. Report bugs. | Suggested by Abductive | Category:Genito-urinary system drug stubs | #UCB_Category 464/590 |
||
| (27 intermediate revisions by 19 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical compound}} |
|||
{{Drugbox |
{{Drugbox |
||
| Verifiedfields = changed |
|||
| ⚫ | |||
| Watchedfields = changed |
|||
| ⚫ | |||
| verifiedrevid = 451555899 |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
<!--Clinical data--> |
|||
| ⚫ | |||
| tradename = |
|||
| ⚫ | |||
| Drugs.com = {{drugs.com|international|denaverine}} |
|||
| ⚫ | |||
| |
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
||
| |
| pregnancy_US = <!-- A / B / C / D / X --> |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| molecular_weight = 383.52 g/mol |
|||
| |
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|||
| pregnancy_US = <!-- A / B / C / D / X --> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
|||
| ⚫ | |||
| routes_of_administration = [[intramuscular injection]], [[suppositories]] |
| routes_of_administration = [[intramuscular injection]], [[suppositories]] |
||
<!--Pharmacokinetic data--> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
<!--Identifiers--> |
|||
| CAS_number_Ref = {{cascite|correct|??}} |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| PubChem = 71130 |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| DrugBank = |
|||
| ChEMBL_Ref = {{ebicite|changed|EBI}} |
|||
| ChEMBL = 1614656 |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = O14NF38MTL |
|||
| KEGG_Ref = {{keggcite|correct|kegg}} |
|||
| ⚫ | |||
| ⚫ | |||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
|||
| ChemSpiderID = 64278 |
|||
<!--Chemical data--> |
|||
| ⚫ | |||
| ⚫ | |||
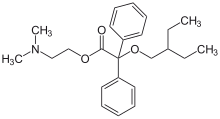
| smiles = CCC(CC)COC(C1=CC=CC=C1)(C2=CC=CC=C2)C(=O)OCCN(C)C |
|||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
|||
| StdInChI = 1S/C24H33NO3/c1-5-20(6-2)19-28-24(21-13-9-7-10-14-21,22-15-11-8-12-16-22)23(26)27-18-17-25(3)4/h7-16,20H,5-6,17-19H2,1-4H3 |
|||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
|||
| StdInChIKey = FPTOUQZVCUIPHY-UHFFFAOYSA-N |
|||
}} |
}} |
||
'''Denaverine''' is an [[antispasmodic]] drug. It was developed in Germany and patented in 1974. '''Denaverine hydrochloride''' is used in veterinary medicine under the trade name '''Sensiblex''' as a muscle relaxant for the [[myometrium]] of cows and dogs during |
'''Denaverine''' is an [[antispasmodic]] drug. It was developed in Germany and patented in 1974. '''Denaverine hydrochloride''' is used in veterinary medicine under the trade name '''Sensiblex''' as a muscle relaxant for the [[myometrium]] of cows and dogs during parturition.<ref>[http://www.ema.europa.eu/pdfs/vet/mrls/030797en.pdf Committee for Veterinary Medicinal Products: Denavering Hydrochloride Summary Report]</ref> Under the trade name '''Spasmalgan''', it has also been used in humans for the treatment of [[urogenital]] and [[gastrointestinal]] spasms.<ref>{{cite book|title=Rote Liste | year = 2005 | veditors = Dootz H, Kuhlmann A, Hoffmann K |at=77 023|edition=2005|isbn=3-87193-306-6|publisher=Editio Cantor|location=Aulendorf|language=German}}</ref> |
||
==Mechanism of action== |
==Mechanism of action== |
||
Denaverine, like [[papaverine]], acts as a [[phosphodiesterase inhibitor]]. Additionally, it has [[anticholinergic]] effects.<ref name="Arzneistoff-Profile">{{cite book|title=Arzneistoff-Profile| |
Denaverine, like [[papaverine]], acts as a [[phosphodiesterase inhibitor]]. Additionally, it has [[anticholinergic]] effects.<ref name="Arzneistoff-Profile">{{cite book|title=Arzneistoff-Profile| veditors = Dinnendahl V, Fricke U |publisher=Govi Pharmazeutischer Verlag|location=Eschborn, Germany|date=2010|edition=23|volume=4|isbn=978-3-7741-9846-3|language=German}}</ref> |
||
==References== |
==References== |
||
| Line 40: | Line 66: | ||
[[Category:Antispasmodics]] |
[[Category:Antispasmodics]] |
||
[[Category: |
[[Category:Dimethylamino compounds]] |
||
[[Category: |
[[Category:Carboxylate esters]] |
||
[[Category:Ethers]] |
[[Category:Ethers]] |
||
{{genito-urinary-drug-stub}} |
{{genito-urinary-drug-stub}} |
||
[[de:Denaverin]] |
|||
Latest revision as of 11:02, 4 February 2023
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | intramuscular injection, suppositories |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 8% (suppositories), 37% (oral solution) |
| Metabolism | mainly hepatic, at least 11 metabolites |
| Elimination half-life | 34 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H33NO3 |
| Molar mass | 383.532 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Denaverine is an antispasmodic drug. It was developed in Germany and patented in 1974. Denaverine hydrochloride is used in veterinary medicine under the trade name Sensiblex as a muscle relaxant for the myometrium of cows and dogs during parturition.[1] Under the trade name Spasmalgan, it has also been used in humans for the treatment of urogenital and gastrointestinal spasms.[2]
Mechanism of action
[edit]Denaverine, like papaverine, acts as a phosphodiesterase inhibitor. Additionally, it has anticholinergic effects.[3]
References
[edit]- ^ Committee for Veterinary Medicinal Products: Denavering Hydrochloride Summary Report
- ^ Dootz H, Kuhlmann A, Hoffmann K, eds. (2005). Rote Liste (in German) (2005 ed.). Aulendorf: Editio Cantor. 77 023. ISBN 3-87193-306-6.
- ^ Dinnendahl V, Fricke U, eds. (2010). Arzneistoff-Profile (in German). Vol. 4 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
