17α-Hydroxypregnenolone: Difference between revisions
m Reverted 1 edit by 136.29.22.212 (talk) to last revision by Maxim Masiutin |
Citation bot (talk | contribs) Add: s2cid. | Use this bot. Report bugs. | Suggested by Abductive | Category:Pregnanes | #UCB_Category 96/278 |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical compound}} |
|||
{{Drugbox |
{{Drugbox |
||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Line 48: | Line 49: | ||
}} |
}} |
||
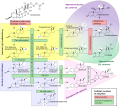
'''17α-Hydroxypregnenolone''' is a [[pregnane]] (C21) [[steroid]] that is obtained by [[hydroxylation]] of [[pregnenolone]] at the C17α position. This step is performed by the [[mitochondria]]l [[cytochrome P450 oxidase|cytochrome P450 enzyme]] 17α-hydroxylase ([[CYP17A1]]) that is present in the [[adrenal]] and [[gonad]]s. Peak levels are reached in humans at the end of [[puberty]] and then decline.<ref>{{cite journal | vauthors = Hill M, Lukác D, Lapcík O, Sulcová J, Hampl R, Pouzar V, Stárka L | title = Age relationships and sex differences in serum levels of pregnenolone and 17-hydroxypregnenolone in healthy subjects | journal = Clinical Chemistry and Laboratory Medicine | volume = 37 | issue = 4 | pages = 439–47 | date = April 1999 | pmid = 10369116 | doi = 10.1515/CCLM.1999.072 }}</ref> High levels are also achieved during [[pregnancy]]. It is also a known [[Neuromodulation|neuromodulator]]. |
'''17α-Hydroxypregnenolone''' is a [[pregnane]] (C21) [[steroid]] that is obtained by [[hydroxylation]] of [[pregnenolone]] at the C17α position. This step is performed by the [[mitochondria]]l [[cytochrome P450 oxidase|cytochrome P450 enzyme]] 17α-hydroxylase ([[CYP17A1]]) that is present in the [[adrenal]] and [[gonad]]s. Peak levels are reached in humans at the end of [[puberty]] and then decline.<ref>{{cite journal | vauthors = Hill M, Lukác D, Lapcík O, Sulcová J, Hampl R, Pouzar V, Stárka L | title = Age relationships and sex differences in serum levels of pregnenolone and 17-hydroxypregnenolone in healthy subjects | journal = Clinical Chemistry and Laboratory Medicine | volume = 37 | issue = 4 | pages = 439–47 | date = April 1999 | pmid = 10369116 | doi = 10.1515/CCLM.1999.072 | s2cid = 41315909 }}</ref> High levels are also achieved during [[pregnancy]]. It is also a known [[Neuromodulation|neuromodulator]]. |
||
==Prohormone== |
==Prohormone== |
||
| Line 60: | Line 61: | ||
== Neurosteroid == |
== Neurosteroid == |
||
17α-hydroxypregnenolone is a known neuromodulator as its acts in the [[central nervous system]]. Specifically, it is known to modulate [[Animal locomotion|locomotion]].<ref>{{cite journal | vauthors = Tsutsui K, Haraguchi S, Vaudry H | title = 7α-Hydroxypregnenolone regulating locomotor behavior identified in the brain and pineal gland across vertebrates | journal = General and Comparative Endocrinology | volume = 265 | pages = 97–105 | date = September 2018 | pmid = 28919448 | doi = 10.1016/j.ygcen.2017.09.014 }}</ref> |
17α-hydroxypregnenolone is a known neuromodulator as its acts in the [[central nervous system]]. Specifically, it is known to modulate [[Animal locomotion|locomotion]].<ref>{{cite journal | vauthors = Tsutsui K, Haraguchi S, Vaudry H | title = 7α-Hydroxypregnenolone regulating locomotor behavior identified in the brain and pineal gland across vertebrates | journal = General and Comparative Endocrinology | volume = 265 | pages = 97–105 | date = September 2018 | pmid = 28919448 | doi = 10.1016/j.ygcen.2017.09.014 | s2cid = 5636071 }}</ref> |
||
== See also == |
== See also == |
||
* [[Congenital adrenal hyperplasia]] |
* [[Congenital adrenal hyperplasia]] |
||
* [[Narave pig]], intersex pigs that have low levels of 17α-Hydroxypregnenolone |
|||
==Additional images== |
==Additional images== |
||
Latest revision as of 19:10, 8 March 2023
 | |
 | |
| Pharmacokinetic data | |
|---|---|
| Metabolism | Adrenal, Gonads |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.239 |
| Chemical and physical data | |
| Formula | C21H32O3 |
| Molar mass | 332.484 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 268 °C (514 °F) |
| |
| |
| | |
17α-Hydroxypregnenolone is a pregnane (C21) steroid that is obtained by hydroxylation of pregnenolone at the C17α position. This step is performed by the mitochondrial cytochrome P450 enzyme 17α-hydroxylase (CYP17A1) that is present in the adrenal and gonads. Peak levels are reached in humans at the end of puberty and then decline.[1] High levels are also achieved during pregnancy. It is also a known neuromodulator.
Prohormone
[edit]17α-Hydroxypregnenolone is considered a prohormone in the formation of dehydroepiandrosterone (DHEA), itself a prohormone of the sex steroids.
This conversion is mediated by the enzyme 17,20 lyase. As such 17α-hydroxypregenolone represents an intermediary in the Δ5 pathway that leads from pregnenolone to DHEA. 17α-Hydroxypregneolone is also converted to 17α-hydroxyprogesterone, a prohormone for glucocorticosteroids and androstenedione through the activity of 3α-hydroxysteroid dehydrogenase.
Clinical use
[edit]Measurements of 17α-hydroxypregnenolone are useful in the diagnosis of certain forms of congenital adrenal hyperplasia.[2] In patients with congenital adrenal hyperplasia due to 3β-hydroxysteroid dehydrogenase deficiency 17α-hydroxypregnenolone is increased, while in patients with congenital adrenal hyperplasia due to 17α-hydroxylase deficiency levels are low to absent.
Neurosteroid
[edit]17α-hydroxypregnenolone is a known neuromodulator as its acts in the central nervous system. Specifically, it is known to modulate locomotion.[3]
See also
[edit]- Congenital adrenal hyperplasia
- Narave pig, intersex pigs that have low levels of 17α-Hydroxypregnenolone
Additional images
[edit]References
[edit]- ^ Hill M, Lukác D, Lapcík O, Sulcová J, Hampl R, Pouzar V, Stárka L (April 1999). "Age relationships and sex differences in serum levels of pregnenolone and 17-hydroxypregnenolone in healthy subjects". Clinical Chemistry and Laboratory Medicine. 37 (4): 439–47. doi:10.1515/CCLM.1999.072. PMID 10369116. S2CID 41315909.
- ^ Riepe FG, Mahler P, Sippell WG, Partsch CJ (September 2002). "Longitudinal study of plasma pregnenolone and 17-hydroxypregnenolone in full-term and preterm neonates at birth and during the early neonatal period". The Journal of Clinical Endocrinology and Metabolism. 87 (9): 4301–6. doi:10.1210/jc.2002-020452. PMID 12213889.
- ^ Tsutsui K, Haraguchi S, Vaudry H (September 2018). "7α-Hydroxypregnenolone regulating locomotor behavior identified in the brain and pineal gland across vertebrates". General and Comparative Endocrinology. 265: 97–105. doi:10.1016/j.ygcen.2017.09.014. PMID 28919448. S2CID 5636071.