Trinitrotriazine: Difference between revisions
Appearance
Content deleted Content added
move PIN |
m →top: Copy edit ▸ Link removed here as the first instance of this term was just linked in my previous edit. Tags: Mobile edit Mobile app edit Android app edit |
||
| (7 intermediate revisions by 6 users not shown) | |||
| Line 43: | Line 43: | ||
}} |
}} |
||
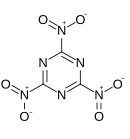
'''Trinitrotriazine''', or '''2,4,6-trinitro-1,3,5-triazine''', is a theoretical [[explosive]] compound. Synthesis of this compound has been elusive despite its simple structure,<ref>G. D. Hartman, R. D. Hartman and J. E. Schwering. ''Tetrahedron Letters''. 1983; 24: 1011.</ref><ref>M. D. Coburn, C. L. Coon, H. H. Hayden and A. R. Mitchell. ''Synthesis''. 1986: 490.</ref> as conventional [[nitration]] of [[triazine]] becomes increasingly more difficult as more nitro groups are added. A successful route would more likely proceed by trimerisation of nitryl cyanide.<ref>Korkin AA. Bartlett RJ. Theoretical Prediction of 2,4,6-Trinitro-1,3,5-triazine (TNTA). A New, Powerful, High-Energy Density Material? ''Journal of the American Chemical Society''. 1996; 118: 12244-12245.</ref> The precursor |
'''Trinitrotriazine''', or '''2,4,6-trinitro-1,3,5-triazine''', is a theoretical [[explosive]] compound. Synthesis of this compound has been elusive despite its simple structure,<ref>G. D. Hartman, R. D. Hartman and J. E. Schwering. ''Tetrahedron Letters''. 1983; 24: 1011.</ref><ref>M. D. Coburn, C. L. Coon, H. H. Hayden and A. R. Mitchell. ''Synthesis''. 1986: 490.</ref> as conventional [[nitration]] of [[triazine]] becomes increasingly more difficult as more nitro groups are added. A successful route would more likely proceed by [[trimerisation]] of [[nitryl cyanide]].<ref>Korkin AA. Bartlett RJ. Theoretical Prediction of 2,4,6-Trinitro-1,3,5-triazine (TNTA). A New, Powerful, High-Energy Density Material? ''Journal of the American Chemical Society''. 1996; 118: 12244-12245.</ref> The precursor nitryl cyanide was first synthesized by Rahm et al. in 2014.<ref>Rahm, M., Bélanger-Chabot, G., Haiges, R. and Christe, K. O. (2014), Nitryl Cyanide, NCNO<sub>2</sub>. Angew. Chem. Int. Ed., 53: 6893–6897</ref> |
||
Trinitrotriazine has a |
Trinitrotriazine has a neutral [[oxygen balance]], potentially making it a very powerful explosive, though calculations predict it would be fairly unstable and inferior to the related compound 3,6-dinitro-1,2,4,5-tetrazine.<ref>Jinshan Li. An Ab Initio Theoretical Study of 2,4,6-Trinitro-1,3,5-Triazine, 3,6-Dinitro-1,2,4,5-Tetrazine, and 2,5,8-Trinitro-Tri-s-Triazine, more commonly known as RDX. ''Propellants, Explosives, Pyrotechnics.'' December 2008; 33(6):443-447.</ref> |
||
==See also== |
==See also== |
||
| Line 56: | Line 56: | ||
[[Category:Triazines]] |
[[Category:Triazines]] |
||
[[Category:Nitro compounds]] |
[[Category:Nitro compounds]] |
||
[[Category:Hypothetical chemical compounds]] |
|||
{{theoretical-chem-stub}} |
|||
Latest revision as of 10:37, 11 March 2023
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2,4,6-Trinitro-1,3,5-triazine | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3N6O6 | |||
| Molar mass | 216.069 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Trinitrotriazine, or 2,4,6-trinitro-1,3,5-triazine, is a theoretical explosive compound. Synthesis of this compound has been elusive despite its simple structure,[1][2] as conventional nitration of triazine becomes increasingly more difficult as more nitro groups are added. A successful route would more likely proceed by trimerisation of nitryl cyanide.[3] The precursor nitryl cyanide was first synthesized by Rahm et al. in 2014.[4]
Trinitrotriazine has a neutral oxygen balance, potentially making it a very powerful explosive, though calculations predict it would be fairly unstable and inferior to the related compound 3,6-dinitro-1,2,4,5-tetrazine.[5]
See also
[edit]- RDX (hexahydro-1,3,5-trinitro-1,3,5-triazine)
References
[edit]- ^ G. D. Hartman, R. D. Hartman and J. E. Schwering. Tetrahedron Letters. 1983; 24: 1011.
- ^ M. D. Coburn, C. L. Coon, H. H. Hayden and A. R. Mitchell. Synthesis. 1986: 490.
- ^ Korkin AA. Bartlett RJ. Theoretical Prediction of 2,4,6-Trinitro-1,3,5-triazine (TNTA). A New, Powerful, High-Energy Density Material? Journal of the American Chemical Society. 1996; 118: 12244-12245.
- ^ Rahm, M., Bélanger-Chabot, G., Haiges, R. and Christe, K. O. (2014), Nitryl Cyanide, NCNO2. Angew. Chem. Int. Ed., 53: 6893–6897
- ^ Jinshan Li. An Ab Initio Theoretical Study of 2,4,6-Trinitro-1,3,5-Triazine, 3,6-Dinitro-1,2,4,5-Tetrazine, and 2,5,8-Trinitro-Tri-s-Triazine, more commonly known as RDX. Propellants, Explosives, Pyrotechnics. December 2008; 33(6):443-447.