Rhodium(III) oxide: Difference between revisions
m →References: adding tmp |
M97uzivatel (talk | contribs) No edit summary |
||
| (30 intermediate revisions by 19 users not shown) | |||
| Line 1: | Line 1: | ||
{{chembox |
{{chembox |
||
|Verifiedfields = changed |
|||
| ⚫ | |||
|Watchedfields = changed |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
<!-- | ImageSize = 200px --> |
|||
| ⚫ | |||
| |
|ImageName = Rhodium(III) oxide |
||
| OtherNames = |
|||
| |
|Section1={{Chembox Identifiers |
||
|CASNo_Ref = {{cascite|correct|??}} |
|||
| |
|CASNo = 12036-35-0 |
||
| ⚫ | |||
|UNII_Ref = {{fdacite|correct|FDA}} |
|||
| ⚫ | |||
|UNII = 6PYI3777JI |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| Formula = Rh<sub>2</sub>O<sub>3</sub> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| |
|Formula = Rh<sub>2</sub>O<sub>3</sub> |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
|Density = 8.20 g/cm<sup>3</sup> |
|||
| ⚫ | |||
| ⚫ | |||
| BoilingPt = |
|||
| ⚫ | |||
|MeltingPtC = 1100 |
|||
| ⚫ | |||
|MagSus = +104.0·10<sup>−6</sup> cm<sup>3</sup>/mol |
|||
| ⚫ | |||
| ⚫ | |||
|Structure_ref = <ref name=Coey1970 /> |
|||
| ⚫ | |||
|SpaceGroup = R{{overline|3}}c |
|||
|LattConst_a = 512.7 pm (hexagonal setting) |
|||
|LattConst_c = 1385.3 pm (hexagonal setting) |
|||
| ⚫ | |||
| ⚫ | |||
|GHSPictograms = {{GHS03}}{{GHS07}} |
|||
|GHSSignalWord = danger |
|||
|HPhrases = {{HPhrases|H315 |H319|H335|H302 + H332 }} |
|||
|PPhrases = {{PPhrases|P301 + P330 + P331 |P312 |P304 + P340 | P302 + P352 |P337 + P313 |P280 |P332 + P313}} |
|||
|EUPhrases = {{EUH-phrases|EUH032 }} |
|||
|GHS_ref = <ref>GHS: [https://www.alfa.com/de/catalog/011814/ Alfa Aesar 011814] SDS (Feb 2021)</ref> |
|||
}} |
}} |
||
| ⚫ | |||
| Coordination = |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| EUClass = not listed |
|||
| ⚫ | |||
}} |
}} |
||
'''Rhodium(III) oxide''' (or '''Rhodium sesquioxide''') is the [[ |
'''Rhodium(III) oxide''' (or '''Rhodium sesquioxide''') is the [[inorganic compound]] with the formula [[Rhodium|Rh<sub>2</sub>]][[Oxide|O<sub>3</sub>]]. It is a gray solid that is insoluble in ordinary solvents. |
||
==Structure== |
==Structure== |
||
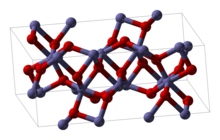
Rh<sub>2</sub>O<sub>3</sub> has been found in two major forms. The hexagonal form |
Rh<sub>2</sub>O<sub>3</sub> has been found in two major forms. The hexagonal form adopts the [[corundum]] structure. It transforms into an [[orthorhombic]] structure when heated above 750 °C.<ref name=Coey1970>{{cite journal | last=Coey | first=J. M. D. | title=The crystal structure of Rh<sub>2</sub>O<sub>3</sub> | journal=Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry | publisher=International Union of Crystallography (IUCr) | volume=26 | issue=11 | date=1970-11-01 | issn=0567-7408 | doi=10.1107/s0567740870005022 | pages=1876–1877}}</ref> |
||
==Production== |
==Production== |
||
Rhodium oxide can be produced via several routes: |
Rhodium oxide can be produced via several routes: |
||
* Treating RhCl<sub>3</sub> with oxygen at high temperatures.<ref>{{cite book|editor=G. Brauer|author=H. L. Grube|chapter=The Platinum Metals|title=Handbook of Preparative Inorganic Chemistry, 2nd Ed.|publisher=Academic Press|year=1963|place=NY|page=1588}}</ref> |
|||
* Rh metal powder is fused with [[potassium hydrogen sulfate]]. Adding [[sodium hydroxide]] results in [[hydrate]]d rhodium oxide, which upon heating converts to Rh<sub>2</sub>O<sub>3</sub>.<ref name=wold> |
* Rh metal powder is fused with [[potassium hydrogen sulfate]]. Adding [[sodium hydroxide]] results in [[hydrate]]d rhodium oxide, which upon heating converts to Rh<sub>2</sub>O<sub>3</sub>.<ref name=wold>{{cite journal | last=Wold | first=Aaron | last2=Arnott | first2=Ronald J. | last3=Croft | first3=William J. | title=The Reaction of Rare Earth Oxides with a High Temperature Form of Rhodium(III) Oxide | journal=Inorganic Chemistry | publisher=American Chemical Society (ACS) | volume=2 | issue=5 | year=1963 | issn=0020-1669 | doi=10.1021/ic50009a023 | pages=972–974}}</ref> |
||
* Rhodium oxide [[thin film]]s can be produced by exposing Rh layer to oxygen plasma.<ref name=apl/> |
* Rhodium oxide [[thin film]]s can be produced by exposing Rh layer to oxygen plasma.<ref name=apl/> |
||
* [[Nanoparticle]]s can be produced by the [[hydrothermal synthesis]].<ref>{{cite journal | last=Mulukutla | first=Ravichandra S. | last2=Asakura | first2=Kiyotaka | last3=Kogure | first3=Toshihiro | last4=Namba | first4=Seitaro | last5=Iwasawa | first5=Yasuhiro | title=Synthesis and characterization of rhodium oxide nanoparticles in mesoporous MCM-41 | journal=Physical Chemistry Chemical Physics | publisher=Royal Society of Chemistry (RSC) | volume=1 | issue=8 | year=1999 | issn=1463-9076 | doi=10.1039/a900588i | pages=2027–2032| bibcode=1999PCCP....1.2027M}}</ref> |
|||
* [[Nanoparticle]]s can be produced by the [[hydrothermal synthesis]].<ref>R. S. Mulukutla "Synthesis and characterization of rhodium oxide nanoparticles in |
|||
mesoporous MCM-41" [http://dx.doi.org/10.1039/a900588i Phys. Chem. Chem. Phys. 1 (1999) 2027]</ref> |
|||
==Physical properties== |
==Physical properties== |
||
Rhodium oxide films behave as a fast two-color [[Electrochromism|electrochromic]] system: Reversible yellow ↔ dark green or yellow ↔ brown-purple color changes are obtained in [[potassium hydroxide|KOH]] solutions by applying voltage ~1 [[volt|V]].<ref>S. |
Rhodium oxide films behave as a fast two-color [[Electrochromism|electrochromic]] system: Reversible yellow ↔ dark green or yellow ↔ brown-purple color changes are obtained in [[potassium hydroxide|KOH]] solutions by applying voltage ~1 [[volt|V]].<ref>{{cite journal | last=Gottesfeld | first=S. | title=The Anodic Rhodium Oxide Film: A Two-Color Electrochromic System | journal=Journal of the Electrochemical Society | publisher=The Electrochemical Society | volume=127 | issue=2 | year=1980 | issn=0013-4651 | doi=10.1149/1.2129654 | page=272}}</ref> |
||
Electrochromic System" [http://link.aip.org/link/?JESOAN/127/272/1 J. Electrochem. Soc. 127 (1980) 272]</ref> |
|||
Rhodium oxide films are transparent and conductive, like [[indium tin oxide]] (ITO) - the common transparent electrode, but Rh<sub>2</sub>O<sub>3</sub> has 0.2 eV lower [[work function]] than ITO. Consequently, deposition of rhodium oxide on ITO improves the carrier injection from ITO thereby improving the electrical properties of [[organic light-emitting diode]]s.<ref name=apl> |
Rhodium oxide films are transparent and conductive, like [[indium tin oxide]] (ITO) - the common transparent electrode, but Rh<sub>2</sub>O<sub>3</sub> has 0.2 eV lower [[work function]] than ITO. Consequently, deposition of rhodium oxide on ITO improves the carrier injection from ITO thereby improving the electrical properties of [[organic light-emitting diode]]s.<ref name=apl>{{cite journal | last=Kim | first=Soo Young | last2=Baik | first2=Jeong Min | last3=Yu | first3=Hak Ki | last4=Kim | first4=Kwang Young | last5=Tak | first5=Yoon-Heung | last6=Lee | first6=Jong-Lam | title=Rhodium-oxide-coated indium tin oxide for enhancement of hole injection in organic light emitting diodes | journal=Applied Physics Letters | publisher=AIP Publishing | volume=87 | issue=7 | date=2005-08-15 | issn=0003-6951 | doi=10.1063/1.2012534 | page=072105| bibcode=2005ApPhL..87g2105K | url=https://scholarworks.unist.ac.kr/handle/201301/7384}}</ref> |
||
==Catalytic properties== |
|||
==Applications== |
|||
Rhodium oxides are [[catalyst]]s for [[hydroformylation]] of alkenes,<ref>{{cite journal|last1=Pino|first1=P.|last2=Botteghi|first2=C.|title=Aldehydes from olefins: cyclohexanecarboxaldehyde|journal=Organic Syntheses|date=1977|volume=57|page=11|doi=10.15227/orgsyn.057.0011}}</ref> [[nitrous oxide|N<sub>2</sub>O]] production from [[nitric oxide|NO]],<ref>{{cite journal | last=Mulukutla | first=Ravichandra S | last2=Shido | first2=Takafumi | last3=Asakura | first3=Kiyotaka | last4=Kogure | first4=Toshihiro | last5=Iwasawa | first5=Yasuhiro | title=Characterization of rhodium oxide nanoparticles in MCM-41 and their catalytic performances for NO–CO reactions in excess O<sub>2</sub> | journal=Applied Catalysis A: General | publisher=Elsevier BV | volume=228 | issue=1–2 | year=2002 | issn=0926-860X | doi=10.1016/s0926-860x(01)00992-9 | pages=305–314}}</ref> and the [[hydrogenation]] of [[carbon monoxide|CO]].<ref>{{cite journal | last=Watson| first=P |first2=G. A. |last2=Somorjai| title=The hydrogenation of carbon monoxide over rhodium oxide surfaces | journal=Journal of Catalysis | publisher=Elsevier BV | volume=72 | issue=2 | year=1981 | issn=0021-9517 | doi=10.1016/0021-9517(81)90018-x | pages=347–363| url=https://escholarship.org/uc/item/861193km}}</ref> |
|||
The major application of rhodium oxides is in [[catalyst]]s, e.g. for the conversion of [[carbon monoxide|CO]]<ref>P. R. Watson and G. A. Somorjai "The hydrogenation of carbon monoxide over rhodium oxide surfaces" [http://dx.doi.org/10.1016/0021-9517(81)90018-X Journal of Catalysis 72 (1981) 347]</ref> or [[nitric oxide|NO]] gases.<ref>R. S. Mulukutla "Characterization of rhodium oxide nanoparticles in MCM-41 and their catalytic performances for NO–CO reactions in excess O2" [http://dx.doi.org/10.1016/S0926-860X(01)00992-9 Applied Catalysis A: 228 (2002) 305]</ref> |
|||
==Safety== |
|||
Conditions/substances to avoid are: extreme [[heat]], [[organic solvent]]s, [[hydrochloric acid]], [[hydrosulfuric acid]] and [[ammonia]]. |
|||
==See also== |
==See also== |
||
*[[Rhodium]] |
*[[Rhodium]] |
||
*[[Rhodium(IV) oxide]] |
*[[Rhodium(IV) oxide]] |
||
*[[Rhodium-platinum oxide]] |
|||
==References== |
==References== |
||
| Line 68: | Line 74: | ||
{{Rhodium compounds}} |
{{Rhodium compounds}} |
||
{{Oxides}} |
{{Oxides}} |
||
{{DEFAULTSORT:Rhodium(Iii) Oxide}} |
|||
[[Category: |
[[Category:Transition metal oxides]] |
||
[[Category:Rhodium compounds]] |
[[Category:Rhodium(III) compounds]] |
||
[[Category:Sesquioxides]] |
[[Category:Sesquioxides]] |
||
[[Category:Chromism]] |
|||
Latest revision as of 17:04, 3 April 2023
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ECHA InfoCard | 100.031.666 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Rh2O3 | |
| Molar mass | 253.8092 g/mol |
| Appearance | dark grey odorless powder |
| Density | 8.20 g/cm3 |
| Melting point | 1,100 °C (2,010 °F; 1,370 K) (decomposes) |
| insoluble | |
| Solubility | insoluble in aqua regia |
| +104.0·10−6 cm3/mol | |
| Structure[1] | |
| hexagonal (corundum) | |
| R3c | |
a = 512.7 pm (hexagonal setting), c = 1385.3 pm (hexagonal setting)
| |
| Hazards | |
| GHS labelling:[2] | |
 
| |
| Danger | |
| H302+H332, H315, H319, H335 | |
| P280, P301+P330+P331, P302+P352, P304+P340, P312, P332+P313, P337+P313 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rhodium(III) oxide (or Rhodium sesquioxide) is the inorganic compound with the formula Rh2O3. It is a gray solid that is insoluble in ordinary solvents.
Structure
[edit]Rh2O3 has been found in two major forms. The hexagonal form adopts the corundum structure. It transforms into an orthorhombic structure when heated above 750 °C.[1]
Production
[edit]Rhodium oxide can be produced via several routes:
- Treating RhCl3 with oxygen at high temperatures.[3]
- Rh metal powder is fused with potassium hydrogen sulfate. Adding sodium hydroxide results in hydrated rhodium oxide, which upon heating converts to Rh2O3.[4]
- Rhodium oxide thin films can be produced by exposing Rh layer to oxygen plasma.[5]
- Nanoparticles can be produced by the hydrothermal synthesis.[6]
Physical properties
[edit]Rhodium oxide films behave as a fast two-color electrochromic system: Reversible yellow ↔ dark green or yellow ↔ brown-purple color changes are obtained in KOH solutions by applying voltage ~1 V.[7]
Rhodium oxide films are transparent and conductive, like indium tin oxide (ITO) - the common transparent electrode, but Rh2O3 has 0.2 eV lower work function than ITO. Consequently, deposition of rhodium oxide on ITO improves the carrier injection from ITO thereby improving the electrical properties of organic light-emitting diodes.[5]
Catalytic properties
[edit]Rhodium oxides are catalysts for hydroformylation of alkenes,[8] N2O production from NO,[9] and the hydrogenation of CO.[10]
See also
[edit]References
[edit]- ^ a b Coey, J. M. D. (1970-11-01). "The crystal structure of Rh2O3". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry. 26 (11). International Union of Crystallography (IUCr): 1876–1877. doi:10.1107/s0567740870005022. ISSN 0567-7408.
- ^ GHS: Alfa Aesar 011814 SDS (Feb 2021)
- ^ H. L. Grube (1963). "The Platinum Metals". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. NY: Academic Press. p. 1588.
- ^ Wold, Aaron; Arnott, Ronald J.; Croft, William J. (1963). "The Reaction of Rare Earth Oxides with a High Temperature Form of Rhodium(III) Oxide". Inorganic Chemistry. 2 (5). American Chemical Society (ACS): 972–974. doi:10.1021/ic50009a023. ISSN 0020-1669.
- ^ a b Kim, Soo Young; Baik, Jeong Min; Yu, Hak Ki; Kim, Kwang Young; Tak, Yoon-Heung; Lee, Jong-Lam (2005-08-15). "Rhodium-oxide-coated indium tin oxide for enhancement of hole injection in organic light emitting diodes". Applied Physics Letters. 87 (7). AIP Publishing: 072105. Bibcode:2005ApPhL..87g2105K. doi:10.1063/1.2012534. ISSN 0003-6951.
- ^ Mulukutla, Ravichandra S.; Asakura, Kiyotaka; Kogure, Toshihiro; Namba, Seitaro; Iwasawa, Yasuhiro (1999). "Synthesis and characterization of rhodium oxide nanoparticles in mesoporous MCM-41". Physical Chemistry Chemical Physics. 1 (8). Royal Society of Chemistry (RSC): 2027–2032. Bibcode:1999PCCP....1.2027M. doi:10.1039/a900588i. ISSN 1463-9076.
- ^ Gottesfeld, S. (1980). "The Anodic Rhodium Oxide Film: A Two-Color Electrochromic System". Journal of the Electrochemical Society. 127 (2). The Electrochemical Society: 272. doi:10.1149/1.2129654. ISSN 0013-4651.
- ^ Pino, P.; Botteghi, C. (1977). "Aldehydes from olefins: cyclohexanecarboxaldehyde". Organic Syntheses. 57: 11. doi:10.15227/orgsyn.057.0011.
- ^ Mulukutla, Ravichandra S; Shido, Takafumi; Asakura, Kiyotaka; Kogure, Toshihiro; Iwasawa, Yasuhiro (2002). "Characterization of rhodium oxide nanoparticles in MCM-41 and their catalytic performances for NO–CO reactions in excess O2". Applied Catalysis A: General. 228 (1–2). Elsevier BV: 305–314. doi:10.1016/s0926-860x(01)00992-9. ISSN 0926-860X.
- ^ Watson, P; Somorjai, G. A. (1981). "The hydrogenation of carbon monoxide over rhodium oxide surfaces". Journal of Catalysis. 72 (2). Elsevier BV: 347–363. doi:10.1016/0021-9517(81)90018-x. ISSN 0021-9517.
