Hydroxyquinol: Difference between revisions
m →Production: Wikilink added |
m →Natural occurrence: cite repair; |
||
| (25 intermediate revisions by 14 users not shown) | |||
| Line 7: | Line 7: | ||
| ImageName = Chemical structure of hydroxyquinol |
| ImageName = Chemical structure of hydroxyquinol |
||
| ImageAlt = Chemical structure of hydroxyquinol |
| ImageAlt = Chemical structure of hydroxyquinol |
||
| |
| PIN = Benzene-1,2,4-triol |
||
| OtherNames = Hydroxyhydroquinone |
| OtherNames = Hydroxyhydroquinone<br>1,2,4-Benzenetriol<br>1,2,4-Trihydroxybenzene<br>Benzene-1,2,4-triol<br>4-Hydroxycatechol<br>2,4-Dihydroxyphenol<br>1,3,4-Benzenetriol<br>1,3,4-Trihydroxybenzene |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| Line 22: | Line 22: | ||
| CASNo_Ref = {{cascite|correct|CAS}}= |
| CASNo_Ref = {{cascite|correct|CAS}}= |
||
| CASNoOther = |
| CASNoOther = |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = 173O8B04RD |
|||
| PubChem = 10787 |
| PubChem = 10787 |
||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
| ChEBI_Ref = {{ebicite|correct|EBI}} |
||
| Line 32: | Line 34: | ||
| Formula = C<sub>6</sub>H<sub>6</sub>O<sub>3</sub> |
| Formula = C<sub>6</sub>H<sub>6</sub>O<sub>3</sub> |
||
| MolarMass = 126.11 g/mol |
| MolarMass = 126.11 g/mol |
||
| Appearance = |
| Appearance = white solid |
||
| Density = |
| Density = |
||
| MeltingPt = |
| MeltingPt = |
||
| Line 39: | Line 41: | ||
}} |
}} |
||
}} |
}} |
||
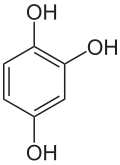
'''Hydroxyquinol''' is an [[organic compound]] with the formula C<sub>6</sub>H<sub>3</sub>(OH)<sub>3</sub>. It is one of three isomeric [[benzenetriol]]s. The compound is a colorless solid that is soluble in water. It reacts with air to give a black insoluble solid.<ref name="Ullmann">{{cite journal|last1=Fiege|first1=Helmut|last2=Heinz-Werner|first2=Voges|last3=Hamamoto|first3=Toshikazu|last4=Umemura|first4=Sumio|last5=Iwata|first5=Tadao|last6=Miki|first6=Hisaya|last7=Fujita|first7=Yasuhiro|last8=Buysch|first8=Hans-Josef|last9=Garbe|first9=Dorothea|last10=Paulus|first10=Wilfried|title=Phenol Derivatives|journal=Ullmann's Encyclopedia of Industrial Chemistry|year=2005|publisher=Wiley-VCH|location=Weinheim, Germany|doi=10.1002/14356007.a19_313|isbn=978-3527306732}}</ref> |
|||
'''Hydroxyquinol''' is a [[benzenetriol]]. |
|||
==Production== |
==Production== |
||
It is prepared industrially by acetylation of [[paraquinone]] with [[acetic anhydride]] followed by hydrolysis of the triacetate.<ref name="Ullmann"/> |
|||
| ⚫ | |||
| ⚫ | Historically, hydroxyquinol was produced by the action of [[potassium hydroxide]] on [[hydroquinone]].<ref>{{cite book|last=Roscoe|first=Henry|title=A treatise on chemistry, Volume 3, Part 3|year=1891|publisher=Macmillan & Co.|location=London|pages=199|url=https://books.google.com/books?id=HEY9AAAAYAAJ&q=hydroxyquinol&pg=PA199}}</ref> It can also be prepared by [[Dehydration reaction|dehydrating]] [[fructose]].<ref>{{cite journal|last1=Luijkx|first1=Gerard|last2=Rantwijk|first2=Fred|last3=Bekkum|first3=Herman|author-link3=Herman van Bekkum|title=Hydrothermal formation of 1,2,4-benzenetriol from 5-hydroxymethyl-2-furaldehyde and D-fructose|journal=Carbohydrate Research|date=1993|volume=242|issue=1|pages=131–139|doi=10.1016/0008-6215(93)80027-C}}</ref><ref>{{cite journal|last1=Srokol|first1=Zbigniew|last2=Anne-Gaëlle|first2=Bouche|last3=Estrik|first3=Anton|last4=Strik|first4=Rob|last5=Maschmeyer|first5=Thomas|last6=Peters|first6=Joop|title=Hydrothermal upgrading of biomass to biofuel; studies on some monosaccharide model compounds|journal=Carbohydrate Research|date=2004|volume=339|issue=10|pages=1717–1726|doi=10.1016/j.carres.2004.04.018|pmid=15220081}}</ref> |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | Hydroxyquinol commonly occurs in nature as a [[biodegradation]] product of [[catechin]], a [[phenol|natural phenol]] found in plants |
||
| ⚫ | |||
Hydroxyquinol is a common intermediate in the biodegradation of many aromatic compounds. These substrates include mono[[chlorophenol]]s, [[dichlorophenol]]s, and more complex species such as the pesticide [[2,4,5-Trichlorophenoxyacetic acid|2,4,5-T]].<ref>{{cite journal|title=Hydroxyquinol pathway for microbial degradation of halogenated aromatic compounds |
|||
| ⚫ | |author1=Travkin, Vasili M. |author2=Solyanikova, Inna P. |author3=Golovleva, Ludmila A. |journal=Journal of Environmental Science and Health, Part B |year=2006|volume=41|issue=8|pages=1361–1382|doi=10.1080/03601230600964159|pmid=17090498|s2cid=36347319}}</ref> Hydroxyquinol commonly occurs in nature as a [[biodegradation]] product of [[catechin]], a [[phenol|natural phenol]] found in plants (e.g. by soil bacteria ''[[Bradyrhizobium japonicum]]'').<ref>{{cite journal|last1=Mahadevan|first1=A.|last2=Waheeta|first2=Hopper|title=Degradation of catechin by Bradyrhizobium japonicum|journal=Biodegradation|date=1997|volume=8|issue=3|pages=159–165|doi=10.1023/A:1008254812074|s2cid=41221044}}</ref> Hydroxyquinol is also a [[metabolite]] in some organisms. For instance, [[Hydroxyquinol 1,2-dioxygenase]] is an [[enzyme]] that uses hydroxyquinol as a [[Enzyme substrate (biology)|substrate]] with [[oxygen]] to produce [[3-hydroxy-cis,cis-muconate]]. |
||
==References== |
==References== |
||
| Line 53: | Line 58: | ||
[[Category:Hydroxyquinols| ]] |
[[Category:Hydroxyquinols| ]] |
||
{{Aromatic-stub}} |
|||
Latest revision as of 13:51, 28 April 2023
| |
| Names | |
|---|---|
| Preferred IUPAC name
Benzene-1,2,4-triol | |
| Other names
Hydroxyhydroquinone
1,2,4-Benzenetriol 1,2,4-Trihydroxybenzene Benzene-1,2,4-triol 4-Hydroxycatechol 2,4-Dihydroxyphenol 1,3,4-Benzenetriol 1,3,4-Trihydroxybenzene | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.797 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H6O3 | |
| Molar mass | 126.11 g/mol |
| Appearance | white solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hydroxyquinol is an organic compound with the formula C6H3(OH)3. It is one of three isomeric benzenetriols. The compound is a colorless solid that is soluble in water. It reacts with air to give a black insoluble solid.[1]
Production
[edit]It is prepared industrially by acetylation of paraquinone with acetic anhydride followed by hydrolysis of the triacetate.[1]
Historically, hydroxyquinol was produced by the action of potassium hydroxide on hydroquinone.[2] It can also be prepared by dehydrating fructose.[3][4]
- C6H12O6 → 3 H2O + C6H6O3
Natural occurrence
[edit]Hydroxyquinol is a common intermediate in the biodegradation of many aromatic compounds. These substrates include monochlorophenols, dichlorophenols, and more complex species such as the pesticide 2,4,5-T.[5] Hydroxyquinol commonly occurs in nature as a biodegradation product of catechin, a natural phenol found in plants (e.g. by soil bacteria Bradyrhizobium japonicum).[6] Hydroxyquinol is also a metabolite in some organisms. For instance, Hydroxyquinol 1,2-dioxygenase is an enzyme that uses hydroxyquinol as a substrate with oxygen to produce 3-hydroxy-cis,cis-muconate.
References
[edit]- ^ a b Fiege, Helmut; Heinz-Werner, Voges; Hamamoto, Toshikazu; Umemura, Sumio; Iwata, Tadao; Miki, Hisaya; Fujita, Yasuhiro; Buysch, Hans-Josef; Garbe, Dorothea; Paulus, Wilfried (2005). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim, Germany: Wiley-VCH. doi:10.1002/14356007.a19_313. ISBN 978-3527306732.
- ^ Roscoe, Henry (1891). A treatise on chemistry, Volume 3, Part 3. London: Macmillan & Co. p. 199.
- ^ Luijkx, Gerard; Rantwijk, Fred; Bekkum, Herman (1993). "Hydrothermal formation of 1,2,4-benzenetriol from 5-hydroxymethyl-2-furaldehyde and D-fructose". Carbohydrate Research. 242 (1): 131–139. doi:10.1016/0008-6215(93)80027-C.
- ^ Srokol, Zbigniew; Anne-Gaëlle, Bouche; Estrik, Anton; Strik, Rob; Maschmeyer, Thomas; Peters, Joop (2004). "Hydrothermal upgrading of biomass to biofuel; studies on some monosaccharide model compounds". Carbohydrate Research. 339 (10): 1717–1726. doi:10.1016/j.carres.2004.04.018. PMID 15220081.
- ^ Travkin, Vasili M.; Solyanikova, Inna P.; Golovleva, Ludmila A. (2006). "Hydroxyquinol pathway for microbial degradation of halogenated aromatic compounds". Journal of Environmental Science and Health, Part B. 41 (8): 1361–1382. doi:10.1080/03601230600964159. PMID 17090498. S2CID 36347319.
- ^ Mahadevan, A.; Waheeta, Hopper (1997). "Degradation of catechin by Bradyrhizobium japonicum". Biodegradation. 8 (3): 159–165. doi:10.1023/A:1008254812074. S2CID 41221044.
