Hydroxyquinol: Difference between revisions
TB Leenders (talk | contribs) Review |
m →Natural occurrence: cite repair; |
||
| (14 intermediate revisions by 12 users not shown) | |||
| Line 7: | Line 7: | ||
| ImageName = Chemical structure of hydroxyquinol |
| ImageName = Chemical structure of hydroxyquinol |
||
| ImageAlt = Chemical structure of hydroxyquinol |
| ImageAlt = Chemical structure of hydroxyquinol |
||
| |
| PIN = Benzene-1,2,4-triol |
||
| OtherNames = Hydroxyhydroquinone |
| OtherNames = Hydroxyhydroquinone<br>1,2,4-Benzenetriol<br>1,2,4-Trihydroxybenzene<br>Benzene-1,2,4-triol<br>4-Hydroxycatechol<br>2,4-Dihydroxyphenol<br>1,3,4-Benzenetriol<br>1,3,4-Trihydroxybenzene |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| Line 22: | Line 22: | ||
| CASNo_Ref = {{cascite|correct|CAS}}= |
| CASNo_Ref = {{cascite|correct|CAS}}= |
||
| CASNoOther = |
| CASNoOther = |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = 173O8B04RD |
|||
| PubChem = 10787 |
| PubChem = 10787 |
||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
| ChEBI_Ref = {{ebicite|correct|EBI}} |
||
| Line 32: | Line 34: | ||
| Formula = C<sub>6</sub>H<sub>6</sub>O<sub>3</sub> |
| Formula = C<sub>6</sub>H<sub>6</sub>O<sub>3</sub> |
||
| MolarMass = 126.11 g/mol |
| MolarMass = 126.11 g/mol |
||
| Appearance = |
| Appearance = white solid |
||
| Density = |
| Density = |
||
| MeltingPt = |
| MeltingPt = |
||
| Line 39: | Line 41: | ||
}} |
}} |
||
}} |
}} |
||
'''Hydroxyquinol''' is an [[organic compound]] with the formula C<sub>6</sub>H<sub>3</sub>(OH)<sub>3</sub>. It is one of three isomeric [[benzenetriol]]s. The compound is a colorless solid that is soluble in water. It reacts with air to give a black insoluble solid.<ref name="Ullmann">{{cite journal|last1=Fiege|first1=Helmut|last2=Heinz-Werner|first2=Voges|last3=Hamamoto|first3=Toshikazu|last4=Umemura|first4=Sumio|last5=Iwata|first5=Tadao|last6=Miki|first6=Hisaya|last7=Fujita|first7=Yasuhiro|last8=Buysch|first8=Hans-Josef|last9=Garbe|first9=Dorothea|last10=Paulus|first10=Wilfried|title=Phenol Derivatives|journal=Ullmann's Encyclopedia of Industrial Chemistry|year=2005|publisher=Wiley-VCH|location=Weinheim, Germany|doi=10.1002/14356007.a19_313|isbn=978-3527306732}}</ref> |
|||
'''Hydroxyquinol''' is a [[benzenetriol]]. It is also known as 1,2,4-Benzenetriol |
|||
== Review == |
|||
1,2,4-benzenetriol is a primary metabolized product by cytochrome P450 from benzene.<ref>{{Cite web|url=http://linkinghub.elsevier.com/retrieve/pii/S0300483X14000171?via=sd&cc=y|title=Elsevier: Article Locator|website=linkinghub.elsevier.com|access-date=2017-03-17}}</ref> 1,2,4-benzenetriol with a triphenolic structure, strongly reacts with molecular oxygen. It has been reported that 1,2,4-benzenetriol induces oxidative DNA damage and breaks DNA strands. |
|||
==Production== |
==Production== |
||
It is prepared industrially by acetylation of [[paraquinone]] with [[acetic anhydride]] followed by hydrolysis of the triacetate.<ref name="Ullmann"/> |
|||
| ⚫ | |||
| ⚫ | |||
== History == |
|||
1,2,4-benzenetriol was first discovered in the 19th century.<ref>{{Cite journal|last=Snyder|first=R|last2=Hedli|first2=C C|date=1996-12-01|title=An overview of benzene metabolism.|url=http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1469747/|journal=Environmental Health Perspectives|volume=104|issue=Suppl 6|pages=1165–1171|issn=0091-6765|pmc=PMC1469747|pmid=9118888}}</ref> During an experiment in 1953 Parke and Williams<ref>{{Cite journal|last=Parke|first=D. V.|last2=Williams|first2=R. T.|date=2017-03-17|title=Studies in detoxication. 49. The metabolism of benzene containing [14C1]benzene|url=http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1268930/|journal=Biochemical Journal|volume=54|issue=2|pages=231–238|issn=0264-6021|pmc=PMC1268930|pmid=13058864}}</ref> reported that upon administering benzene to rabbits they could recover phenol, catechol, hydroquinone, 1,2,4-benzenetriol, transtrans-muconic acid, and L-phenylmercapturic acid in the urine, exhaled air, feces and tissue of the rabbits. They went on to suggest that benzene toxicity, i.e., benzeneinduced bone marrow depression, might be caused by some of these metabolites. |
|||
== Metabolism == |
|||
1,2,4-benzenetriol is a product of benzene metabolism in humans.<ref>{{Cite journal|last=Snyder|first=R|last2=Hedli|first2=C C|date=1996-12-01|title=An overview of benzene metabolism.|url=http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1469747/|journal=Environmental Health Perspectives|volume=104|issue=Suppl 6|pages=1165–1171|issn=0091-6765|pmc=PMC1469747|pmid=9118888}}</ref> The hydroxyl radical formed from H202 can hydroxylate benzene to yield phenol. When benzene oxide is the first product, it can rearrange nonenzymatically to form phenol. Alternatively, benzene oxide can be hydrated via epoxide hydrolase to yield 1,2-benzene dihydrodiol, which can in turn be oxidized via dihydrodiol dehydrogenase to form catechol. The reaction of benzene oxide with glutathione catalyzed by glutathione S-transferase leads to the formation of the premercapturic acid. It is likely that benzene oxide or its oxepin are precursors to ring opening . Phenol can be further hydroxylated to form hydroquinone or catechol. In theory, 1,2,4-benzenetriol may be formed by the hydroxylation of either hydroquinone or catechol. |
|||
[[File:Benzene metabolism.png|thumb|Metabolism of benzene in to p-Benzoquinone, 1,2,4-Trihydroxybenzene and o-Benzoquinone]] |
|||
| ⚫ | |||
| ⚫ | Hydroxyquinol commonly occurs in nature as a [[biodegradation]] product of [[catechin]], a [[phenol|natural phenol]] found in plants |
||
| ⚫ | Historically, hydroxyquinol was produced by the action of [[potassium hydroxide]] on [[hydroquinone]].<ref>{{cite book|last=Roscoe|first=Henry|title=A treatise on chemistry, Volume 3, Part 3|year=1891|publisher=Macmillan & Co.|location=London|pages=199|url=https://books.google.com/books?id=HEY9AAAAYAAJ&q=hydroxyquinol&pg=PA199}}</ref> It can also be prepared by [[Dehydration reaction|dehydrating]] [[fructose]].<ref>{{cite journal|last1=Luijkx|first1=Gerard|last2=Rantwijk|first2=Fred|last3=Bekkum|first3=Herman|author-link3=Herman van Bekkum|title=Hydrothermal formation of 1,2,4-benzenetriol from 5-hydroxymethyl-2-furaldehyde and D-fructose|journal=Carbohydrate Research|date=1993|volume=242|issue=1|pages=131–139|doi=10.1016/0008-6215(93)80027-C}}</ref><ref>{{cite journal|last1=Srokol|first1=Zbigniew|last2=Anne-Gaëlle|first2=Bouche|last3=Estrik|first3=Anton|last4=Strik|first4=Rob|last5=Maschmeyer|first5=Thomas|last6=Peters|first6=Joop|title=Hydrothermal upgrading of biomass to biofuel; studies on some monosaccharide model compounds|journal=Carbohydrate Research|date=2004|volume=339|issue=10|pages=1717–1726|doi=10.1016/j.carres.2004.04.018|pmid=15220081}}</ref> |
||
== Toxicity == |
|||
Although not much is known of 1,2,4-Benzenetriol in a pure it does have some identifications that it has hazardous effects on the human body.<ref>{{Cite web|url=https://pubchem.ncbi.nlm.nih.gov/compound/1_2_4-benzenetriol#section=Top|title=1,2,4-BENZENETRIOL {{!}} C6H6O3 - PubChem|last=Pubchem|website=pubchem.ncbi.nlm.nih.gov|language=en|access-date=2017-03-17}}</ref> 1,2,4-benzene triol causes irritation to the skin, irritation to the eyes, serious eye damage and it may cause respiratory irritation. Cases of these effect have occurred but no documented cases are known. |
|||
| ⚫ | |||
=== DNA damage === |
|||
1,2,4-Benzenetriol caused strong DNA damage.<ref>{{Cite journal|last=Kawanishi|first=Shosuke|last2=Inoue|first2=Sumiko|last3=Kawanishi|first3=Michiko|date=1989-01-01|title=Human DNA Damage Induced by 1,2,4-Benzenetriol, a Benzene Metabolite|url=http://cancerres.aacrjournals.org/content/49/1/164|journal=Cancer Research|language=en|volume=49|issue=1|pages=164–168|issn=0008-5472|pmid=2908843}}</ref> |
|||
| ⚫ | |||
== Ways of detection == |
|||
Hydroxyquinol is a common intermediate in the biodegradation of many aromatic compounds. These substrates include mono[[chlorophenol]]s, [[dichlorophenol]]s, and more complex species such as the pesticide [[2,4,5-Trichlorophenoxyacetic acid|2,4,5-T]].<ref>{{cite journal|title=Hydroxyquinol pathway for microbial degradation of halogenated aromatic compounds |
|||
1,2,4-benzenetriol can be detected by means of high performance liquid chromatography.<ref>{{Cite journal|last=Inoue|first=O.|last2=Seiji|first2=K.|last3=Nakatsuka|first3=H.|last4=Watanabe|first4=T.|last5=Yin|first5=S.|last6=Li|first6=G. L.|last7=Cai|first7=S. X.|last8=Jin|first8=C.|last9=Ikeda|first9=M.|date=1989-08-01|title=Excretion of 1,2,4-benzenetriol in the urine of workers exposed to benzene.|url=http://oem.bmj.com/content/46/8/559|journal=Occupational and Environmental Medicine|language=en|volume=46|issue=8|pages=559–565|doi=10.1136/oem.46.8.559|issn=1351-0711|pmid=2775675}}</ref> This is mostely being performed on samples from organisms that have been exposed to benzene. |
|||
| ⚫ | |author1=Travkin, Vasili M. |author2=Solyanikova, Inna P. |author3=Golovleva, Ludmila A. |journal=Journal of Environmental Science and Health, Part B |year=2006|volume=41|issue=8|pages=1361–1382|doi=10.1080/03601230600964159|pmid=17090498|s2cid=36347319}}</ref> Hydroxyquinol commonly occurs in nature as a [[biodegradation]] product of [[catechin]], a [[phenol|natural phenol]] found in plants (e.g. by soil bacteria ''[[Bradyrhizobium japonicum]]'').<ref>{{cite journal|last1=Mahadevan|first1=A.|last2=Waheeta|first2=Hopper|title=Degradation of catechin by Bradyrhizobium japonicum|journal=Biodegradation|date=1997|volume=8|issue=3|pages=159–165|doi=10.1023/A:1008254812074|s2cid=41221044}}</ref> Hydroxyquinol is also a [[metabolite]] in some organisms. For instance, [[Hydroxyquinol 1,2-dioxygenase]] is an [[enzyme]] that uses hydroxyquinol as a [[Enzyme substrate (biology)|substrate]] with [[oxygen]] to produce [[3-hydroxy-cis,cis-muconate]]. |
||
==References== |
==References== |
||
| Line 72: | Line 58: | ||
[[Category:Hydroxyquinols| ]] |
[[Category:Hydroxyquinols| ]] |
||
{{Aromatic-stub}} |
|||
Latest revision as of 13:51, 28 April 2023
| |
| Names | |
|---|---|
| Preferred IUPAC name
Benzene-1,2,4-triol | |
| Other names
Hydroxyhydroquinone
1,2,4-Benzenetriol 1,2,4-Trihydroxybenzene Benzene-1,2,4-triol 4-Hydroxycatechol 2,4-Dihydroxyphenol 1,3,4-Benzenetriol 1,3,4-Trihydroxybenzene | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.797 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H6O3 | |
| Molar mass | 126.11 g/mol |
| Appearance | white solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
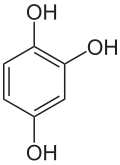
Hydroxyquinol is an organic compound with the formula C6H3(OH)3. It is one of three isomeric benzenetriols. The compound is a colorless solid that is soluble in water. It reacts with air to give a black insoluble solid.[1]
Production
[edit]It is prepared industrially by acetylation of paraquinone with acetic anhydride followed by hydrolysis of the triacetate.[1]
Historically, hydroxyquinol was produced by the action of potassium hydroxide on hydroquinone.[2] It can also be prepared by dehydrating fructose.[3][4]
- C6H12O6 → 3 H2O + C6H6O3
Natural occurrence
[edit]Hydroxyquinol is a common intermediate in the biodegradation of many aromatic compounds. These substrates include monochlorophenols, dichlorophenols, and more complex species such as the pesticide 2,4,5-T.[5] Hydroxyquinol commonly occurs in nature as a biodegradation product of catechin, a natural phenol found in plants (e.g. by soil bacteria Bradyrhizobium japonicum).[6] Hydroxyquinol is also a metabolite in some organisms. For instance, Hydroxyquinol 1,2-dioxygenase is an enzyme that uses hydroxyquinol as a substrate with oxygen to produce 3-hydroxy-cis,cis-muconate.
References
[edit]- ^ a b Fiege, Helmut; Heinz-Werner, Voges; Hamamoto, Toshikazu; Umemura, Sumio; Iwata, Tadao; Miki, Hisaya; Fujita, Yasuhiro; Buysch, Hans-Josef; Garbe, Dorothea; Paulus, Wilfried (2005). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim, Germany: Wiley-VCH. doi:10.1002/14356007.a19_313. ISBN 978-3527306732.
- ^ Roscoe, Henry (1891). A treatise on chemistry, Volume 3, Part 3. London: Macmillan & Co. p. 199.
- ^ Luijkx, Gerard; Rantwijk, Fred; Bekkum, Herman (1993). "Hydrothermal formation of 1,2,4-benzenetriol from 5-hydroxymethyl-2-furaldehyde and D-fructose". Carbohydrate Research. 242 (1): 131–139. doi:10.1016/0008-6215(93)80027-C.
- ^ Srokol, Zbigniew; Anne-Gaëlle, Bouche; Estrik, Anton; Strik, Rob; Maschmeyer, Thomas; Peters, Joop (2004). "Hydrothermal upgrading of biomass to biofuel; studies on some monosaccharide model compounds". Carbohydrate Research. 339 (10): 1717–1726. doi:10.1016/j.carres.2004.04.018. PMID 15220081.
- ^ Travkin, Vasili M.; Solyanikova, Inna P.; Golovleva, Ludmila A. (2006). "Hydroxyquinol pathway for microbial degradation of halogenated aromatic compounds". Journal of Environmental Science and Health, Part B. 41 (8): 1361–1382. doi:10.1080/03601230600964159. PMID 17090498. S2CID 36347319.
- ^ Mahadevan, A.; Waheeta, Hopper (1997). "Degradation of catechin by Bradyrhizobium japonicum". Biodegradation. 8 (3): 159–165. doi:10.1023/A:1008254812074. S2CID 41221044.
