Engeletin: Difference between revisions
Appearance
Content deleted Content added
Updating {{chembox}} (changes to watched fields) per Chem/Drugbox validation (report errors or bugs) |
add semisystematic name |
||
| (6 intermediate revisions by 6 users not shown) | |||
| Line 3: | Line 3: | ||
| verifiedrevid = 440125457 |
| verifiedrevid = 440125457 |
||
| Name = Engeletin |
| Name = Engeletin |
||
| ImageFile = Engeletin. |
| ImageFile = Engeletin.svg |
||
| ImageSize = 200px |
| ImageSize = 200px |
||
| ImageName = Chemical structure of engeletin |
| ImageName = Chemical structure of engeletin |
||
| ImageAlt = Chemical structure of engeletin |
| ImageAlt = Chemical structure of engeletin |
||
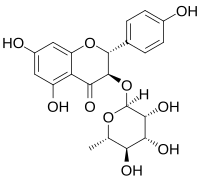
| IUPACName = ( |
| IUPACName = (2''R'',3''R'')-4′,5,7-Trihydroxy-3-(α-<small>L</small>-rhamnopyranosyloxy)flavan-4-one |
||
| PIN = (2''R'',3''R'')-5,7-Dihydroxy-2-(4-hydroxyphenyl)-3-{[(2''S'',3''R'',4''R'',5''R'',6''S'')-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}-4''H''-1-benzopyran-4-one |
|||
| OtherNames = [[dihydrokaempferol]] 3-[[rhamnoside]]<br>Engelitin |
| OtherNames = [[dihydrokaempferol]] 3-[[rhamnoside]]<br>Engelitin |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| Line 19: | Line 20: | ||
}} |
}} |
||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| |
| C=21 | H=22 | O=10 |
||
| MolarMass = 434.39 g/mol |
|||
| Appearance = |
| Appearance = |
||
| Density = |
| Density = |
||
| Line 30: | Line 30: | ||
| MainHazards = |
| MainHazards = |
||
| FlashPt = |
| FlashPt = |
||
| AutoignitionPt = |
| AutoignitionPt = |
||
| |
| GHS_ref = <!-- no GHS data found in PubChem Dec2021 --> |
||
| SPhrases = <!-- {{S1/2}}, {{S9}}, {{S16}}, {{S26}}, {{S36/37/39}}, {{S45}}, {{S61}} etc. --> |
|||
}} |
}} |
||
}} |
}} |
||
'''Engeletin''' is a phenolic compound found in [[wine]]<ref>{{ cite journal | |
'''Engeletin''' is a [[flavanonol]] [[rhamnoside]], a phenolic compound found in [[wine]]<ref>{{ cite journal |author1=Trousdale, E. K. |author2=Singleton, V. L. | title = Astilbin and Engeletin in Grapes and Wine | journal = Phytochemistry | year = 1983 | volume = 22 | issue = 2 | pages = 619–20 | doi = 10.1016/0031-9422(83)83072-6 }}</ref> and isolated from the bark of ''[[Hymenaea martiana]]''.<ref>{{ cite journal |author1=Carneiro, E. |author2=Calixto, J. B. |author3=Monache, F. D. |author4=Yunes, R. A. | title = Isolation, Chemical Identification and Pharmacological Evaluation of Eucryphin, Astilbin and Engelitin Obtained from the Bark of ''Hymenaea martiana'' | journal = Pharmaceutical Biology | year = 1993 | volume = 31 | issue = 1 | pages = 38–46 | doi = 10.3109/13880209309082916 }}</ref> |
||
== See also == |
== See also == |
||
| Line 47: | Line 46: | ||
[[Category:Flavonoid rhamnosides]] |
[[Category:Flavonoid rhamnosides]] |
||
{{Natural-phenol-stub}} |
|||
{{aromatic-stub}} |
|||
Latest revision as of 01:23, 1 May 2023
| |
| Names | |
|---|---|
| IUPAC name
(2R,3R)-4′,5,7-Trihydroxy-3-(α-L-rhamnopyranosyloxy)flavan-4-one
| |
| Preferred IUPAC name
(2R,3R)-5,7-Dihydroxy-2-(4-hydroxyphenyl)-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Other names | |
| Identifiers | |
| |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C21H22O10 | |
| Molar mass | 434.397 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Engeletin is a flavanonol rhamnoside, a phenolic compound found in wine[1] and isolated from the bark of Hymenaea martiana.[2]
See also
[edit]References
[edit]- ^ Trousdale, E. K.; Singleton, V. L. (1983). "Astilbin and Engeletin in Grapes and Wine". Phytochemistry. 22 (2): 619–20. doi:10.1016/0031-9422(83)83072-6.
- ^ Carneiro, E.; Calixto, J. B.; Monache, F. D.; Yunes, R. A. (1993). "Isolation, Chemical Identification and Pharmacological Evaluation of Eucryphin, Astilbin and Engelitin Obtained from the Bark of Hymenaea martiana". Pharmaceutical Biology. 31 (1): 38–46. doi:10.3109/13880209309082916.
