Carbenoid: Difference between revisions
m Reverted 1 edit by 2405:204:610F:C899:FC70:9C0C:6D97:80F9 (talk) to last revision by Magic links bot. (TW) |
m added link |
||
| (3 intermediate revisions by 3 users not shown) | |||
| Line 5: | Line 5: | ||
This complex reacts with an [[alkene]] to form a [[cyclopropane]] just as a carbene would do. |
This complex reacts with an [[alkene]] to form a [[cyclopropane]] just as a carbene would do. |
||
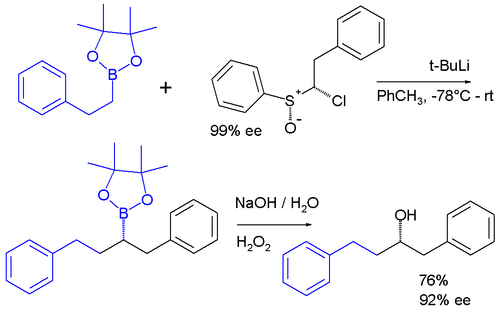
Carbenoids appear as intermediates in many other reactions. In one system a carbenoid chloroalkyllithium reagent is prepared in situ from a [[sulfoxide]] and [[ |
Carbenoids appear as intermediates in many other reactions. In one system a carbenoid chloroalkyllithium reagent is prepared in situ from a [[sulfoxide]] and [[Tert-Butyllithium|t-BuLi]] which reacts the boronic ester to give an ate complex. The ate complex undergoes a 1,2-metallate rearrangement to give the homologated product, which is then further oxidised to a secondary alcohol.<ref>Iterative Stereospecific Reagent-Controlled Homologation of Pinacol Boronates by Enantioenriched -Chloroalkyllithium Reagents Paul R. Blakemore and Matthew S. Burge [[J. Am. Chem. Soc.]]; '''2007'''; 129(11) pp 3068 - 3069; (Communication) {{doi|10.1021/ja068808s}}</ref> |
||
:[[Image:CarbenoidApplication.png|500px|Insertion of carbenoid into C-B bond]] |
:[[Image:CarbenoidApplication.png|500px|Insertion of carbenoid into C-B bond]] |
||
The [[enantiopurity]] of the chiral sulfoxide is preserved in the ultimate product after oxidation of the boronic ester to the [[alcohol]] indicating that a true carbene was never involved in the sequence. |
The [[enantiopurity]] of the chiral sulfoxide is preserved in the ultimate product after oxidation of the boronic ester to the [[Alcohol (chemistry)|alcohol]] indicating that a true carbene was never involved in the sequence. |
||
==See also== |
==See also== |
||
Latest revision as of 15:43, 5 May 2023
In chemistry a carbenoid is a reactive intermediate that shares reaction characteristics with a carbene.[1] In the Simmons–Smith reaction the carbenoid intermediate is a zinc / iodine complex that takes the form of
- I-CH2-Zn-I
This complex reacts with an alkene to form a cyclopropane just as a carbene would do.
Carbenoids appear as intermediates in many other reactions. In one system a carbenoid chloroalkyllithium reagent is prepared in situ from a sulfoxide and t-BuLi which reacts the boronic ester to give an ate complex. The ate complex undergoes a 1,2-metallate rearrangement to give the homologated product, which is then further oxidised to a secondary alcohol.[2]
The enantiopurity of the chiral sulfoxide is preserved in the ultimate product after oxidation of the boronic ester to the alcohol indicating that a true carbene was never involved in the sequence.
See also
[edit]- The silicon pendant: Silylenoids
References
[edit]- ^ Organic Chemistry john McMurry Brooks /Cole Publishing Company 1988 ISBN 0-534-07968-7
- ^ Iterative Stereospecific Reagent-Controlled Homologation of Pinacol Boronates by Enantioenriched -Chloroalkyllithium Reagents Paul R. Blakemore and Matthew S. Burge J. Am. Chem. Soc.; 2007; 129(11) pp 3068 - 3069; (Communication) doi:10.1021/ja068808s