Tetrahydroxy-1,4-benzoquinone bisoxalate: Difference between revisions
Appearance
Content deleted Content added
m punc |
fix mistake |
||
| (6 intermediate revisions by 6 users not shown) | |||
| Line 6: | Line 6: | ||
| ImageSize1 = 200 |
| ImageSize1 = 200 |
||
| ImageAlt1 = Tetrahydroxy-1,4-benzoquinone bisoxalate molecule |
| ImageAlt1 = Tetrahydroxy-1,4-benzoquinone bisoxalate molecule |
||
| PIN = Benzo[1,2-''b'':4,5-''b''′]bis([1,4]dioxine)hexone |
|||
| IUPACName = |
|||
| OtherNames = |
| OtherNames = Benzo[1,2-b:4,5-b']bis-p-dioxin-2,3,5,7,8,10-hexone (8CI) |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| CASNo = |
| CASNo = 20068-66-0 |
||
| PubChem = |
| PubChem = 85762080 |
||
| SMILES = O=C1C(OC2=O)=C(OC2=O)C(C3=C1OC(C(O3)=O)=O)=O }} |
| SMILES = O=C1C(OC2=O)=C(OC2=O)C(C3=C1OC(C(O3)=O)=O)=O }} |
||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| Line 29: | Line 29: | ||
The compound was first described by [[H. S. Verter]], [[H. Porter]], and [[R. Dominic]] in 1968. It was obtained by reacting tetrahydroxy-1,4-benzoquinone with [[oxalyl chloride]] in [[tetrahydrofuran]]. It is a yellow solid that can be crystallized as a tetrahydrofuran [[solvate]], but could not be prepared in pure form.<ref name=verter> |
The compound was first described by [[H. S. Verter]], [[H. Porter]], and [[R. Dominic]] in 1968. It was obtained by reacting tetrahydroxy-1,4-benzoquinone with [[oxalyl chloride]] in [[tetrahydrofuran]]. It is a yellow solid that can be crystallized as a tetrahydrofuran [[solvate]], but could not be prepared in pure form.<ref name=verter> |
||
H. S. Verter, H. Porter, and R. Dominic (Verter, Porter and Dominic, 1968), ''A new carbon oxide: synthesis of tetrahydroxybenzoquinone bisoxalate''. Chemical Communications |
H. S. Verter, H. Porter, and R. Dominic (Verter, Porter and Dominic, 1968), ''A new carbon oxide: synthesis of tetrahydroxybenzoquinone bisoxalate''. Chemical Communications, p. 973b–974. {{doi|10.1039/C1968000973b}} |
||
</ref> |
</ref> |
||
| Line 41: | Line 41: | ||
{{Oxides of carbon}} |
{{Oxides of carbon}} |
||
{{chem-stub}} |
|||
[[Category:Oxocarbons]] |
[[Category:Oxocarbons]] |
||
[[Category:1,4-Benzoquinones]] |
[[Category:1,4-Benzoquinones]] |
||
[[Category: |
[[Category:Oxalate esters]] |
||
[[Category:Oxygen heterocycles]] |
[[Category:Oxygen heterocycles]] |
||
[[Category:Heterocyclic compounds |
[[Category:Heterocyclic compounds with 3 rings]] |
||
Latest revision as of 19:13, 7 May 2023

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Benzo[1,2-b:4,5-b′]bis([1,4]dioxine)hexone | |
| Other names
Benzo[1,2-b:4,5-b']bis-p-dioxin-2,3,5,7,8,10-hexone (8CI)
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C10O10 | |
| Molar mass | 280.00 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
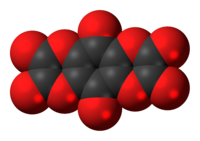
Tetrahydroxy-1,4-benzoquinone bisoxalate is a chemical compound, an oxide of carbon with formula C
10O
10. Its molecule consists of a 1,4-benzoquinone core with the four hydrogen atoms replaced by two oxalate groups. It can be seen as a fourfold ester of tetrahydroxy-1,4-benzoquinone and oxalic acid.
The compound was first described by H. S. Verter, H. Porter, and R. Dominic in 1968. It was obtained by reacting tetrahydroxy-1,4-benzoquinone with oxalyl chloride in tetrahydrofuran. It is a yellow solid that can be crystallized as a tetrahydrofuran solvate, but could not be prepared in pure form.[1]
See also
[edit]- Tetrahydroxy-1,4-benzoquinone biscarbonate
- Hexahydroxybenzene trisoxalate
- Hexahydroxybenzene triscarbonate
References
[edit]- ^ H. S. Verter, H. Porter, and R. Dominic (Verter, Porter and Dominic, 1968), A new carbon oxide: synthesis of tetrahydroxybenzoquinone bisoxalate. Chemical Communications, p. 973b–974. doi:10.1039/C1968000973b
