Rumenic acid: Difference between revisions
Updating {{chembox}} (changes to verified fields - updated 'CASNo_Ref', 'Verifiedfields') per Chem/Drugbox validation (report errors or bugs) |
m Open access bot: doi added to citation with #oabot. |
||
| (22 intermediate revisions by 17 users not shown) | |||
| Line 1: | Line 1: | ||
{{chembox |
{{chembox |
||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Watchedfields = changed |
|||
| verifiedrevid = 447321268 |
| verifiedrevid = 447321268 |
||
| Name = Rumenic acid |
| Name = Rumenic acid |
||
| Line 6: | Line 7: | ||
| ImageSize = 200px |
| ImageSize = 200px |
||
| ImageName = Rumenic acid |
| ImageName = Rumenic acid |
||
| |
| PIN = (9''Z'',11''E'')-Octadeca-9,11-dienoic acid |
||
| OtherNames = Bovinic acid; C9-T11 acid |
|||
| Section1 = {{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| CASNo_Ref = {{cascite| |
| CASNo_Ref = {{cascite|correct|CAS}} |
||
| CASNo = |
| CASNo = 2540-56-9 |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = 46JZW3MR59 |
|||
| SMILES = CCCCCC\C=C/C=C/CCCCCCCC(=O)O |
| SMILES = CCCCCC\C=C/C=C/CCCCCCCC(=O)O |
||
| PubChem = 5280644 |
|||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
|||
| ChemSpiderID = 4444245 |
|||
| InChI = 1/C18H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h7-10H,2-6,11-17H2,1H3,(H,19,20)/b8-7+,10-9- |
|||
| InChIKey = JBYXPOFIGCOSSB-GOJKSUSPBK |
|||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
|||
| StdInChI = 1S/C18H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h7-10H,2-6,11-17H2,1H3,(H,19,20)/b8-7+,10-9- |
|||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
|||
| StdInChIKey = JBYXPOFIGCOSSB-GOJKSUSPSA-N |
|||
| RTECS = |
|||
| MeSHName = |
|||
| ChEBI_Ref = {{ebicite|changed|EBI}} |
|||
| ChEBI = 32798 |
|||
| KEGG_Ref = {{keggcite|changed|kegg}} |
|||
| KEGG = C04056 |
|||
}} |
}} |
||
| Section2 = {{Chembox Properties |
| Section2 = {{Chembox Properties |
||
| |
| C=18|H=32|O=2 |
||
| MolarMass = 280.445 g/mol |
|||
| Density = |
| Density = |
||
| MeltingPt = |
| MeltingPt = |
||
| Line 21: | Line 39: | ||
}} |
}} |
||
'''Rumenic acid''', also known as '''bovinic acid''', is a [[conjugated linoleic acid]] (CLA) found in the fat of [[ruminant]]s and in [[dairy product]]s. It is an [[Essential fatty acid#Nomenclature and terminology|omega-7]] [[trans |
'''Rumenic acid''', also known as '''bovinic acid''', is a [[conjugated linoleic acid]] (CLA) found in the fat of [[ruminant]]s and in [[dairy product]]s. It is an [[Essential fatty acid#Nomenclature and terminology|omega-7]] [[trans fatty acid]]. Its lipid shorthand name is cis-9, trans-11 18:2 acid. The name was proposed by Kramer ''et al.'' in 1998.<ref name=Kramer>{{cite journal |vauthors=Kramer J, Parodi P, Jensen R, Mossoba M, Yurawecz M, Adlof R |title=Rumenic acid: a proposed common name for the major conjugated linoleic acid isomer found in natural products |journal=Lipids |volume=33 |issue=8 |page=835 |year=1998 |pmid=9727617 |doi=10.1007/s11745-998-0279-6|s2cid=10693714 }}</ref> It can be considered as the principal dietary form, accounting for as much as 85-90% of the total [[Conjugated linoleic acid|CLA]] content in dairy products.<ref name=Cyberlipid>{{cite web |
||
The name was proposed by Kramer ''et al.'' in 1998.<ref name=Kramer>{{cite journal |author=Kramer J, Parodi P, Jensen R, Mossoba M, Yurawecz M, Adlof R |title=Rumenic acid: a proposed common name for the major conjugated linoleic acid isomer found in natural products |journal=Lipids |volume=33 |issue=8 |pages=835 |year=1998 |pmid=9727617 |doi=10.1007/s11745-998-0279-6}}</ref> It is formed along with [[vaccenic acid]] by biohydrogenation of dietary [[polyunsaturated fatty acid]]s in the [[rumen]].<ref name=Destaillats>{{cite journal |
|||
| ⚫ | |||
| ⚫ | |||
|author=F. Destaillats, E. Buyukpamukcu, P.-A. Golay, F. Dionisi and F. Giuffrida}}</ref> |
|||
It can be considered as the principal dietary form, accounting for as much as 85-90% of the total [[CLA]] content in dairy products.<ref name=Cyberlipid>{{cite web |
|||
| url=http://www.cyberlipid.org/fa/acid0003.htm |
| url=http://www.cyberlipid.org/fa/acid0003.htm |
||
| title= Polyenoic Fatty Acids |
| title= Polyenoic Fatty Acids |
||
| author=Cyberlipid| accessdate=2007-01-17}}</ref> |
| author=Cyberlipid| accessdate=2007-01-17}}</ref> |
||
==Biosynthesis and biotransformations== |
|||
==Biological properties== |
|||
Rumenic acid is produced from [[vaccenic acid]] by the action of unsaturase enzymes.<ref>{{cite journal |doi=10.1093/ajcn/76.3.504|title=Bioconversion of vaccenic acid to conjugated linoleic acid in humans |year=2002 |last1=Turpeinen |first1=Anu M. |last2=Mutanen |first2=Marja |last3=Aro |first3=Antti |last4=Salminen |first4=Irma |last5=Basu |first5=Samar |last6=Palmquist |first6=Donald L. |last7=Griinari |first7=J Mikko |journal=The American Journal of Clinical Nutrition |volume=76 |issue=3 |pages=504–510 |pmid=12197992 |doi-access=free }}</ref> Rumenic acid is converted back to vaccenic acid en route to [[stearic acid]] |
|||
:{{main|Conjugated linoleic acid}} |
|||
Laboratory studies indicate that rumenic acid shows [[anticarcinogen]]ic properties.<ref name=lock>{{cite journal |
|||
==Further reading== |
|||
|url =http://jn.nutrition.org/cgi/content/abstract/134/10/2698| journal=J Nutr|volume=134|pages=2698–704 |
|||
{{cite journal |
|||
|title= The anticarcinogenic effect of trans-11 18:1 is dependent on its conversion to cis-9, trans-11 CLA by delta9-desaturase in rats |
|||
| ⚫ | |||
|author= Lock AL, Corl BA, Barbano DM, Bauman DE, Ip C.|accessdate=2007-01-15 |
|||
| ⚫ | |||
|pmid= 15465769 |
|||
|author=F. Destaillats |
|||
|issue =10 |
|||
|author2=E. Buyukpamukcu |
|||
|date = October 1, 2004 }}</ref> |
|||
|author3=P.-A. Golay |
|||
|author4=F. Dionisi |
|||
|author5=F. Giuffrida |
|||
|name-list-style=amp |
|||
|doi= 10.3168/jds.S0022-0302(05)72705-3 |
|||
|page= 449 |pmid=15653508|doi-access=free |
|||
}} |
|||
==References== |
==References== |
||
{{Reflist}} |
{{Reflist}} |
||
{{Fatty acids}} |
|||
{{DEFAULTSORT:Rumenic Acid}} |
{{DEFAULTSORT:Rumenic Acid}} |
||
[[Category:Fatty acids]] |
[[Category:Fatty acids]] |
||
[[Category:Alkenoic acids]] |
|||
Latest revision as of 18:53, 12 August 2023
| |
| Names | |
|---|---|
| Preferred IUPAC name
(9Z,11E)-Octadeca-9,11-dienoic acid | |
| Other names
Bovinic acid; C9-T11 acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H32O2 | |
| Molar mass | 280.452 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
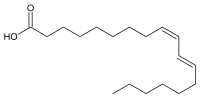
Rumenic acid, also known as bovinic acid, is a conjugated linoleic acid (CLA) found in the fat of ruminants and in dairy products. It is an omega-7 trans fatty acid. Its lipid shorthand name is cis-9, trans-11 18:2 acid. The name was proposed by Kramer et al. in 1998.[1] It can be considered as the principal dietary form, accounting for as much as 85-90% of the total CLA content in dairy products.[2]
Biosynthesis and biotransformations
[edit]Rumenic acid is produced from vaccenic acid by the action of unsaturase enzymes.[3] Rumenic acid is converted back to vaccenic acid en route to stearic acid
Further reading
[edit]F. Destaillats; E. Buyukpamukcu; P.-A. Golay; F. Dionisi & F. Giuffrida (2005). "Letter to the Editor: Vaccenic and Rumenic Acids, A Distinct Feature of Ruminant Fats". Journal of Dairy Science. 88 (449): 449. doi:10.3168/jds.S0022-0302(05)72705-3. PMID 15653508.
References
[edit]- ^ Kramer J, Parodi P, Jensen R, Mossoba M, Yurawecz M, Adlof R (1998). "Rumenic acid: a proposed common name for the major conjugated linoleic acid isomer found in natural products". Lipids. 33 (8): 835. doi:10.1007/s11745-998-0279-6. PMID 9727617. S2CID 10693714.
- ^ Cyberlipid. "Polyenoic Fatty Acids". Retrieved 2007-01-17.
- ^ Turpeinen, Anu M.; Mutanen, Marja; Aro, Antti; Salminen, Irma; Basu, Samar; Palmquist, Donald L.; Griinari, J Mikko (2002). "Bioconversion of vaccenic acid to conjugated linoleic acid in humans". The American Journal of Clinical Nutrition. 76 (3): 504–510. doi:10.1093/ajcn/76.3.504. PMID 12197992.
