Odanacatib: Difference between revisions
Appearance
Content deleted Content added
Add information that development was discontinued. |
consistent citation formatting |
||
| (22 intermediate revisions by 17 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical compound}} |
|||
{{Drugbox |
{{Drugbox |
||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Watchedfields = changed |
|||
| verifiedrevid = 443444475 |
| verifiedrevid = 443444475 |
||
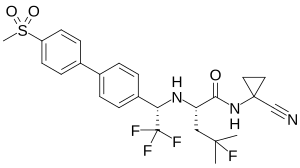
| IUPAC_name = ''N''-(1- |
| IUPAC_name = ''N''-(1-Cyanocyclopropyl)-4-fluoro-''N''<sup>2</sup>-<nowiki/>{(1''S'')-2,2,2-trifluoro-1-[4'-(methylsulfonyl)biphenyl-4-yl]ethyl}-<small>L</small>-leucinamide |
||
| image = Odanacatib |
| image = Odanacatib corrected.svg |
||
| width = 300 |
|||
<!--Clinical data--> |
<!--Clinical data--> |
||
| tradename = |
| tradename = |
||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
||
| pregnancy_US = <!-- A / B |
| pregnancy_US = <!-- A / B/ C / D / X --> |
||
| pregnancy_category = |
| pregnancy_category = |
||
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> |
| legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> |
||
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> |
| legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> |
||
| legal_UK = <!-- GSL, P, POM, CD, or Class A, B, C --> |
| legal_UK = <!-- GSL, P, POM, CD, or Class A, B, C --> |
||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
||
| legal_status = |
| legal_status = Development terminated |
||
| routes_of_administration = |
| routes_of_administration = [[Oral administration|By mouth]] |
||
<!--Pharmacokinetic data--> |
<!--Pharmacokinetic data--> |
||
| bioavailability = |
| bioavailability = |
||
| protein_bound = |
| protein_bound = |
||
| metabolism = |
| metabolism = |
||
| elimination_half-life = |
| elimination_half-life = |
||
| excretion = |
| excretion = |
||
| Line 28: | Line 31: | ||
| CAS_number_Ref = {{cascite|correct|??}} |
| CAS_number_Ref = {{cascite|correct|??}} |
||
| CAS_number = 603139-19-1 |
| CAS_number = 603139-19-1 |
||
| ATC_prefix = |
| ATC_prefix = None |
||
| ATC_suffix = |
| ATC_suffix = |
||
| ATC_supplemental = |
| ATC_supplemental = |
||
| PubChem = 10152654 |
| PubChem = 10152654 |
||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
||
| DrugBank = |
| DrugBank = |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
| UNII_Ref = {{fdacite|correct|FDA}} |
||
| UNII = N673F6W2VH |
| UNII = N673F6W2VH |
||
| KEGG_Ref = {{keggcite|changed|kegg}} |
|||
| KEGG = D08955 |
|||
| ChEMBL_Ref = {{ebicite|changed|EBI}} |
| ChEMBL_Ref = {{ebicite|changed|EBI}} |
||
| ChEMBL = 481611 |
| ChEMBL = 481611 |
||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
||
| ChemSpiderID |
| ChemSpiderID = 8328162 |
||
<!--Chemical data--> |
<!--Chemical data--> |
||
| chemical_formula = |
| chemical_formula = |
||
| C=25 | H=27 | F=4 | N=3 | O=3 | S=1 |
| C=25 | H=27 | F=4 | N=3 | O=3 | S=1 |
||
| molecular_weight = 525.56 g/mol |
|||
| smiles = CC(C)(CC(C(=O)NC1(CC1)C#N)NC(C2=CC=C(C=C2)C3=CC=C(C=C3)S(=O)(=O)C)C(F)(F)F)F |
| smiles = CC(C)(CC(C(=O)NC1(CC1)C#N)NC(C2=CC=C(C=C2)C3=CC=C(C=C3)S(=O)(=O)C)C(F)(F)F)F |
||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
||
| StdInChI |
| StdInChI = 1S/C25H27F4N3O3S/c1-23(2,26)14-20(22(33)32-24(15-30)12-13-24)31-21(25(27,28)29)18-6-4-16(5-7-18)17-8-10-19(11-9-17)36(3,34)35/h4-11,20-21,31H,12-14H2,1-3H3,(H,32,33)/t20-,21-/m0/s1 |
||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
||
| StdInChIKey |
| StdInChIKey = FWIVDMJALNEADT-SFTDATJTSA-N |
||
| synonyms = <small>(2''S'')-''N''-(1- |
| synonyms = <small>(2''S'')-''N''-(1-Cyanocyclopropyl)-4-fluoro-4-methyl-2-<nowiki/>{[(1''S'')-2,2,2-trifluoro-1-<nowiki/>{4'-(methanesulfonyl)-[1,1'-biphenyl]-4-yl}ethyl]amino}pentanamide</small> |
||
}} |
}} |
||
'''Odanacatib''' ([[International Nonproprietary Name|pINN]]; codenamed '''MK-0822''') is an investigational treatment for [[osteoporosis]] and [[bone metastasis]].<ref>{{Cite journal |
|||
| doi = 10.1097/SPC.0b013e32830baea9 |
|||
| title = Cathepsin K inhibitors as treatment of bone metastasis |
|||
| year = 2008 |
|||
| last1 = Le Gall | first1 = C. L. |
|||
| last2 = Bonnelye | first2 = E. |
|||
| last3 = Clézardin | first3 = P. |
|||
| journal = Current Opinion in Supportive and Palliative Care |
|||
| volume = 2 |
|||
| pages = 218–22| pmid = 18685424 |
|||
| issue = 3 |
|||
}}</ref> It is an [[enzyme inhibitor|inhibitor]] of [[cathepsin K]],<ref name="pmid18226527">{{cite journal |vauthors=Gauthier JY, Chauret N, Cromlish W, etal |title=The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K |journal=Bioorg. Med. Chem. Lett. |volume=18 |issue=3 |pages=923–8 |date=February 2008 |pmid=18226527 |doi=10.1016/j.bmcl.2007.12.047 |url=}}</ref> an [[enzyme]] involved in [[bone resorption]]. |
|||
'''Odanacatib''' ([[International nonproprietary name|INN]];<ref name="INN">{{cite journal |title=International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary names: List 60 | journal = WHO Drug Information | volume = 33 | issue= 3 | date = 2008 |url=https://www.who.int/medicines/publications/druginformation/innlists/RL60.pdf | archive-url = https://web.archive.org/web/20131026150609/https://www.who.int/medicines/publications/druginformation/innlists/RL60.pdf | archive-date = 26 October 2013 |publisher=World Health Organization|access-date=11 November 2016|page=239 }}</ref> codenamed '''MK-0822''') is an investigational treatment for [[osteoporosis]] and [[bone metastasis]].<ref>{{cite journal | vauthors = Le Gall C, Bonnelye E, Clézardin P | title = Cathepsin K inhibitors as treatment of bone metastasis | journal = Current Opinion in Supportive and Palliative Care | volume = 2 | issue = 3 | pages = 218–222 | date = September 2008 | pmid = 18685424 | doi = 10.1097/SPC.0b013e32830baea9 | s2cid = 5834581 }}</ref> It is an [[enzyme inhibitor|inhibitor]] of [[cathepsin K]],<ref name="pmid18226527">{{cite journal | vauthors = Gauthier JY, Chauret N, Cromlish W, Desmarais S, Duong LT, Falgueyret JP, Kimmel DB, Lamontagne S, Léger S, LeRiche T, Li CS, Massé F, McKay DJ, Nicoll-Griffith DA, Oballa RM, Palmer JT, Percival MD, Riendeau D, Robichaud J, Rodan GA, Rodan SB, Seto C, Thérien M, Truong VL, Venuti MC, Wesolowski G, Young RN, Zamboni R, Black WC | display-authors = 6 | title = The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K | journal = Bioorganic & Medicinal Chemistry Letters | volume = 18 | issue = 3 | pages = 923–928 | date = February 2008 | pmid = 18226527 | doi = 10.1016/j.bmcl.2007.12.047 }}</ref> an [[enzyme]] involved in [[bone resorption]]. |
|||
| ⚫ | |||
| url=http://www.reuters.com/article/2014/05/06/us-merck-research-idUSKBN0DM14K20140506 |
|||
| title=Merck to seek approval of osteoporosis drug, cites safety risks |
|||
| work=Reuters |
|||
| date=2014-05-06}} |
|||
</ref> |
|||
| ⚫ | The drug was developed by [[Merck & Co.]] The [[clinical trial#Phase III|phase III clinical trial]] for this medicine was stopped early after a review showed it was highly effective and had a good safety profile. Merck announced in 2014 that it would apply for regulatory approval in 2015.<ref>{{cite web | vauthors = Pierson R | date = 15 September 2014 |title=Merck osteoporosis drug passes trial, but side effects hover |website=[[Reuters]] |archive-url=https://web.archive.org/web/20210711172651/https://www.reuters.com/article/us-merck-osteoporosis-idUSKBN0HA1Y820140915 |archive-date=2021-07-11 |url-status=live |url=https://www.reuters.com/article/us-merck-osteoporosis-idUSKBN0HA1Y820140915}}</ref> |
||
| ⚫ | In 2016, Merck discontinued development of |
||
| ⚫ | In 2016, Merck discontinued development of odanacatib and announced it would not seek regulatory approval after analysis discovered an increased risk of [[stroke]].<ref>{{cite news | url=http://www.businesswire.com/news/home/20160902005107/en/Merck-Update-Odanacatib-Development-Program | title=Merck Provides Update on Odanacatib Development Program | work=Business Wire | date=2016-09-02 | access-date=2016-09-30 | archive-url=https://web.archive.org/web/20160903172200/http://www.businesswire.com/news/home/20160902005107/en/Merck-Update-Odanacatib-Development-Program | archive-date=2016-09-03 | url-status=live}}</ref> |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
{{Reflist}} |
{{Reflist}} |
||
{{Drugs for treatment of bone diseases}} |
{{Drugs for treatment of bone diseases}} |
||
[[Category: |
[[Category:Abandoned drugs]] |
||
[[Category:Cyclopropanes]] |
[[Category:Cyclopropanes]] |
||
[[Category:Trifluoromethyl compounds]] |
|||
[[Category:Organofluorides]] |
|||
[[Category:Biphenyls]] |
|||
[[Category:Nitriles]] |
|||
[[Category:Enzyme inhibitors]] |
|||
{{musculoskeletal-drug-stub}} |
{{musculoskeletal-drug-stub}} |
||
Latest revision as of 17:27, 28 October 2023
 | |
| Clinical data | |
|---|---|
| Other names | (2S)-N-(1-Cyanocyclopropyl)-4-fluoro-4-methyl-2-{[(1S)-2,2,2-trifluoro-1-{4'-(methanesulfonyl)-[1,1'-biphenyl]-4-yl}ethyl]amino}pentanamide |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.207.747 |
| Chemical and physical data | |
| Formula | C25H27F4N3O3S |
| Molar mass | 525.56 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Odanacatib (INN;[1] codenamed MK-0822) is an investigational treatment for osteoporosis and bone metastasis.[2] It is an inhibitor of cathepsin K,[3] an enzyme involved in bone resorption.
The drug was developed by Merck & Co. The phase III clinical trial for this medicine was stopped early after a review showed it was highly effective and had a good safety profile. Merck announced in 2014 that it would apply for regulatory approval in 2015.[4]
In 2016, Merck discontinued development of odanacatib and announced it would not seek regulatory approval after analysis discovered an increased risk of stroke.[5]
This drug was developed at Merck Frosst in Montreal.
References
[edit]- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary names: List 60" (PDF). WHO Drug Information. 33 (3). World Health Organization: 239. 2008. Archived from the original (PDF) on 26 October 2013. Retrieved 11 November 2016.
- ^ Le Gall C, Bonnelye E, Clézardin P (September 2008). "Cathepsin K inhibitors as treatment of bone metastasis". Current Opinion in Supportive and Palliative Care. 2 (3): 218–222. doi:10.1097/SPC.0b013e32830baea9. PMID 18685424. S2CID 5834581.
- ^ Gauthier JY, Chauret N, Cromlish W, Desmarais S, Duong LT, Falgueyret JP, et al. (February 2008). "The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K". Bioorganic & Medicinal Chemistry Letters. 18 (3): 923–928. doi:10.1016/j.bmcl.2007.12.047. PMID 18226527.
- ^ Pierson R (15 September 2014). "Merck osteoporosis drug passes trial, but side effects hover". Reuters. Archived from the original on 2021-07-11.
- ^ "Merck Provides Update on Odanacatib Development Program". Business Wire. 2016-09-02. Archived from the original on 2016-09-03. Retrieved 2016-09-30.
