Methoxydienone: Difference between revisions
Tomzzz212004 (talk | contribs) No edit summary |
m Moving Category:Androgens and anabolic steroids to Category:Anabolic–androgenic steroids per Wikipedia:Categories for discussion/Log/2023 October 29#Category:Androgens and anabolic steroids |
||
| (One intermediate revision by one other user not shown) | |||
| Line 71: | Line 71: | ||
[[Category:Abandoned drugs]] |
[[Category:Abandoned drugs]] |
||
[[Category:Androgen ethers]] |
[[Category:Androgen ethers]] |
||
[[Category: |
[[Category:Anabolic–androgenic steroids]] |
||
[[Category:Dienes]] |
[[Category:Dienes]] |
||
[[Category:Estranes]] |
[[Category:Estranes]] |
||
| Line 80: | Line 80: | ||
{{Steroid-stub}} |
{{Steroid-stub}} |
||
{{Genito-urinary-drug-stub}} |
{{Genito-urinary-drug-stub}} |
||
[[Category:Science-related lists]] |
|||
[[Category:Chemistry articles by quality]] |
[[Category:Chemistry articles by quality]] |
||
Latest revision as of 17:46, 11 November 2023
 | |
| Clinical data | |
|---|---|
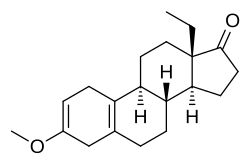
| Other names | Methoxygonadiene; 3-Methoxy-17-dehydro-18-methyl-19-nor-δ2,5(10)-testosterone; 13β-Ethyl-3-methoxygona-2,5(10)-dien-17-one; 18-Methyl-19-nor-δ2,5(10)-epiandrosterone 3-methyl ether |
| Routes of administration | By mouth |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H28O2 |
| Molar mass | 300.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
This article relies largely or entirely on a single source. (January 2022) |
Methoxydienone, also known as methoxygonadiene, as well as 3-methoxy-17-dehydro-18-methyl-19-nor-δ2,5(10)-testosterone or 13β-ethyl-3-methoxygona-2,5(10)-dien-17-one, is a synthetic anabolic-androgenic steroid (AAS) and progestogen of the 19-nortestosterone group related to levonorgestrel which was never marketed.[1] It was synthesized in the 1960s and 1970s by chemist Herchel Smith and his colleagues while they were developing progestins for use in oral contraceptives.[1] The drug is a potent anabolic when administered via injection with an anabolic:androgenic ratio of approximately 54:27 relative to testosterone propionate and 90:625 relative to nandrolone.[1] Methoxydienone is not 17α-alkylated (instead featuring a ketone at the C17 position) and no data exist regarding its oral activity in humans.[1] It has been sold on the Internet as a designer steroid.[1]
See also
[edit]References
[edit]
