Methoxydienone: Difference between revisions
m Open access bot: doi added to citation with #oabot. |
m Moving Category:Androgens and anabolic steroids to Category:Anabolic–androgenic steroids per Wikipedia:Categories for discussion/Log/2023 October 29#Category:Androgens and anabolic steroids |
||
| (6 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Steroid}} |
|||
{{Drugbox |
{{Drugbox |
||
| Verifiedfields = |
| Verifiedfields = |
||
| Line 53: | Line 54: | ||
| StdInChIKey = PQMRKLSVUBRLLQ-XSYGEPLQSA-N |
| StdInChIKey = PQMRKLSVUBRLLQ-XSYGEPLQSA-N |
||
}} |
}} |
||
{{One source|date=January 2022}} |
|||
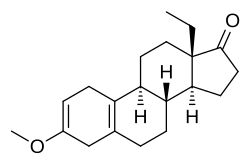
'''Methoxydienone''', also known as '''methoxygonadiene''', as well as '''3-methoxy-17-dehydro-18-methyl-19-nor-δ<sup>2,5(10)</sup>-testosterone''' or '''13β-ethyl-3-methoxygona-2,5(10)-dien-17-one''', is a [[synthetic compound|synthetic]] [[anabolic-androgenic steroid]] (AAS) and [[progestogen]] of the [[19-nortestosterone]] group related to [[levonorgestrel]] which was never marketed.<ref name="pmid25684733">{{cite journal | vauthors = Rahnema CD, Crosnoe LE, Kim ED | title = Designer steroids - over-the-counter supplements and their androgenic component: review of an increasing problem | journal = Andrology | volume = 3 | issue = 2 | pages = 150–5 | year = 2015 | pmid = 25684733 | doi = 10.1111/andr.307 | s2cid = 6999218 | doi-access = free }}</ref> It was [[chemical synthesis|synthesized]] in the 1960s and 1970s by [[chemist]] [[Herchel Smith]] and his colleagues while they were developing [[progestin]]s for use in [[oral contraceptive]]s.<ref name="pmid25684733" /> The drug is a potent [[anabolic]] when administered via [[injection (medicine)|injection]] with an [[anabolic:androgenic ratio]] of approximately 54:27 relative to [[testosterone propionate]] and 90:625 relative to [[nandrolone]].<ref name="pmid25684733" /> Methoxydienone is not [[17α-alkylated anabolic steroid|17α-alkylated]] (instead featuring a [[ketone]] at the C17 position) and no data exist regarding its [[oral administration|oral activity]] in humans.<ref name="pmid25684733" /> It has been sold on the [[Internet]] as a [[designer steroid]].<ref name="pmid25684733" /> |
'''Methoxydienone''', also known as '''methoxygonadiene''', as well as '''3-methoxy-17-dehydro-18-methyl-19-nor-δ<sup>2,5(10)</sup>-testosterone''' or '''13β-ethyl-3-methoxygona-2,5(10)-dien-17-one''', is a [[synthetic compound|synthetic]] [[anabolic-androgenic steroid]] (AAS) and [[progestogen]] of the [[19-nortestosterone]] group related to [[levonorgestrel]] which was never marketed.<ref name="pmid25684733">{{cite journal | vauthors = Rahnema CD, Crosnoe LE, Kim ED | title = Designer steroids - over-the-counter supplements and their androgenic component: review of an increasing problem | journal = Andrology | volume = 3 | issue = 2 | pages = 150–5 | year = 2015 | pmid = 25684733 | doi = 10.1111/andr.307 | s2cid = 6999218 | doi-access = free }}</ref> It was [[chemical synthesis|synthesized]] in the 1960s and 1970s by [[chemist]] [[Herchel Smith]] and his colleagues while they were developing [[progestin]]s for use in [[oral contraceptive]]s.<ref name="pmid25684733" /> The drug is a potent [[anabolic]] when administered via [[injection (medicine)|injection]] with an [[anabolic:androgenic ratio]] of approximately 54:27 relative to [[testosterone propionate]] and 90:625 relative to [[nandrolone]].<ref name="pmid25684733" /> Methoxydienone is not [[17α-alkylated anabolic steroid|17α-alkylated]] (instead featuring a [[ketone]] at the C17 position) and no data exist regarding its [[oral administration|oral activity]] in humans.<ref name="pmid25684733" /> It has been sold on the [[Internet]] as a [[designer steroid]].<ref name="pmid25684733" /> |
||
| Line 70: | Line 71: | ||
[[Category:Abandoned drugs]] |
[[Category:Abandoned drugs]] |
||
[[Category:Androgen ethers]] |
[[Category:Androgen ethers]] |
||
[[Category: |
[[Category:Anabolic–androgenic steroids]] |
||
[[Category:Dienes]] |
[[Category:Dienes]] |
||
[[Category:Estranes]] |
[[Category:Estranes]] |
||
| Line 79: | Line 80: | ||
{{Steroid-stub}} |
{{Steroid-stub}} |
||
{{Genito-urinary-drug-stub}} |
{{Genito-urinary-drug-stub}} |
||
[[Category:Chemistry articles by quality]] |
|||
Latest revision as of 17:46, 11 November 2023
 | |
| Clinical data | |
|---|---|
| Other names | Methoxygonadiene; 3-Methoxy-17-dehydro-18-methyl-19-nor-δ2,5(10)-testosterone; 13β-Ethyl-3-methoxygona-2,5(10)-dien-17-one; 18-Methyl-19-nor-δ2,5(10)-epiandrosterone 3-methyl ether |
| Routes of administration | By mouth |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H28O2 |
| Molar mass | 300.442 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
This article relies largely or entirely on a single source. (January 2022) |
Methoxydienone, also known as methoxygonadiene, as well as 3-methoxy-17-dehydro-18-methyl-19-nor-δ2,5(10)-testosterone or 13β-ethyl-3-methoxygona-2,5(10)-dien-17-one, is a synthetic anabolic-androgenic steroid (AAS) and progestogen of the 19-nortestosterone group related to levonorgestrel which was never marketed.[1] It was synthesized in the 1960s and 1970s by chemist Herchel Smith and his colleagues while they were developing progestins for use in oral contraceptives.[1] The drug is a potent anabolic when administered via injection with an anabolic:androgenic ratio of approximately 54:27 relative to testosterone propionate and 90:625 relative to nandrolone.[1] Methoxydienone is not 17α-alkylated (instead featuring a ketone at the C17 position) and no data exist regarding its oral activity in humans.[1] It has been sold on the Internet as a designer steroid.[1]
See also
[edit]References
[edit]
