Xenon fluoride nitrate: Difference between revisions
NoonIcarus (talk | contribs) |
No edit summary |
||
| (11 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
{{Chembox |
|||
| ⚫ | '''Xenon fluoride nitrate''', also known as '''fluoroxenonium nitrate''', is the chemical compound with formula FXeONO<sub>2</sub>.<ref name="Moran2010">{{cite journal|last=Moran|first=Matthew D.|author2=David S. Brock|author3=Hélène P. A. Mercier|author4=Gary J. Schrobilgen|year=2010|title=Xe3OF3+, a Precursor to a Noble-Gas Nitrate; Syntheses and Structural Characterizations of FXeONO2, XeF2·HNO3, and XeF2·N2O4|journal=Journal of the American Chemical Society|volume=132|issue=39|pages=13823–13839|doi=10.1021/ja105618w|issn=0002-7863|pmid=20843046}}</ref><ref name="Atta-ur-Rahman2006">{{cite book|last=Atta-ur-Rahman |
||
<!-- Images --> |
|||
| ImageFile = Xenon fluoride nitrate.svg |
|||
| ImageSize = 150px |
|||
| ImageAlt = |
|||
<!-- Names --> |
|||
| IUPACName = |
|||
| OtherNames = |
|||
<!-- Sections --> |
|||
| Section1 = {{Chembox Identifiers |
|||
| CASNo = 128970-72-9 |
|||
| PubChem = |
|||
| SMILES = [O-][N+](=O)O[Xe]F |
|||
| StdInChI=1S/FNO3Xe/c1-6-5-2(3)4 |
|||
| StdInChIKey=YCLGDVQFVLWCLS-UHFFFAOYSA-N |
|||
}} |
|||
| Section2 = {{Chembox Properties |
|||
| F = 1 | N = 1 | O = 3 | Xe = 1 |
|||
| Appearance = |
|||
| Density = |
|||
| MeltingPt = |
|||
| BoilingPt = |
|||
| Solubility = |
|||
}} |
|||
| Section3 = {{Chembox Hazards |
|||
| MainHazards = |
|||
| FlashPt = |
|||
| AutoignitionPt = |
|||
}} |
|||
}} |
|||
| ⚫ | '''Xenon fluoride nitrate''', also known as '''fluoroxenonium nitrate''', is the chemical compound with formula FXeONO<sub>2</sub>.<ref name="Moran2010">{{cite journal|last=Moran|first=Matthew D.|author2=David S. Brock|author3=Hélène P. A. Mercier|author4=Gary J. Schrobilgen|year=2010|title=Xe3OF3+, a Precursor to a Noble-Gas Nitrate; Syntheses and Structural Characterizations of FXeONO2, XeF2·HNO3, and XeF2·N2O4|journal=Journal of the American Chemical Society|volume=132|issue=39|pages=13823–13839|doi=10.1021/ja105618w|issn=0002-7863|pmid=20843046}}</ref><ref name="Atta-ur-Rahman2006">{{cite book|last=Atta-ur-Rahman|url=https://books.google.com/books?id=vkcLOFEo9xIC&pg=PA78|title=Advances in Organic Synthesis: Modern Organofluorine Chemistry-Synthetic Aspects|date=2006-01-01|publisher=Bentham Science Publishers|isbn=9781608051984|page=78|access-date=5 October 2014}}</ref> |
||
== Synthesis == |
== Synthesis == |
||
This compound is formed via the reaction:<ref name="MoranM2007">{{cite book|last=Moran|first=Matthew D.|url=https://macsphere.mcmaster.ca/bitstream/11375/14167/1/fulltext.pdf|title=Synthesis and Structural Characterization of new Xenon(II) Compounds and the Use of a Xenon(II) Cation as an Oxidant for the Preparation of Halogenated Hydrocarbons|publisher=McMaster University|year=2007|pages=42, 99–145| |
This compound is formed via the reaction:<ref name="MoranM2007">{{cite book|last=Moran|first=Matthew D.|url=https://macsphere.mcmaster.ca/bitstream/11375/14167/1/fulltext.pdf|title=Synthesis and Structural Characterization of new Xenon(II) Compounds and the Use of a Xenon(II) Cation as an Oxidant for the Preparation of Halogenated Hydrocarbons|publisher=McMaster University|year=2007|pages=42, 99–145|access-date=4 Oct 2014}}</ref> |
||
: [FXeOXeFXeF][AsF<sub>6</sub>] + 2NO<sub>2</sub>F → FXeONO<sub>2</sub> + NO<sub>2</sub>AsF<sub>6</sub>. |
: [FXeOXeFXeF][AsF<sub>6</sub>] + 2NO<sub>2</sub>F → FXeONO<sub>2</sub> + NO<sub>2</sub>AsF<sub>6</sub>. |
||
| Line 20: | Line 51: | ||
== Properties == |
== Properties == |
||
FXeONO<sub>2</sub> is a white crystalline material.<ref name="MoranM2007" /> The [[space group]] of the crystals is P2<sub>1</sub>/c, which is [[monoclinic]]. The unit cell contains four molecules with a total volume of 386.6 Å<sup>3</sup>. The unit cell dimensions are a = 4.6663 Å, b = 8.799 Å c = 9.415 Å, with non-perpendicular angle β = 90.325°.<ref name="MoranM2007" /> With a molecular weight of 212.3, the crystal has density 3.648. (These measurements at -173 °C.)<ref name="MoranM2007" /> |
FXeONO<sub>2</sub> is a white crystalline material.<ref name="MoranM2007" /> The [[space group]] of the crystals is P2<sub>1</sub>/c, which is [[monoclinic]]. The unit cell contains four molecules with a total volume of 386.6 Å<sup>3</sup>. The unit cell dimensions are a = 4.6663 Å, b = 8.799 Å c = 9.415 Å, with non-perpendicular angle β = 90.325°.<ref name="MoranM2007" /> With a molecular weight of 212.3, the crystal has density 3.648. (These measurements at -173 °C.)<ref name="MoranM2007" /> |
||
| ⚫ | The bond lengths in the molecule are 1.992 Å for Xe–F, 2.126 Å for Xe–O, 1.36 Å for O–NO<sub>2</sub>, 1.199 for N–O<sub>cis</sub> and 1.224 Å for N–O<sub>trans</sub>.<ref name="MoranM2007" /> The bond angles are 177.6° for F–Xe–O, 114.7° for Xe-O-N, 114.5° for (Xe)O–N–O<sub>cis</sub>, 118.4° for (Xe)O–N–O<sub>trans</sub> and 127.1° for O<sub>cis</sub>–N–O<sub>trans</sub>.<ref name="MoranM2007" /> The bond lengths and angles on the xenon atom are similar to that in FXeOSO<sub>2</sub>F and FXeOTeF<sub>5</sub>, indicating a polar oxygen bond. The Xe–O–N angle is larger than those in halogen nitrates, which indicates a lower bond density for the Xe–O bond. The N–O<sub>cis</sub> bond length is longer than the N–O<sub>trans</sub> bond length, opposite to other halogen nitrates.<ref name="MoranM2007" /> |
||
[[File:FXeNO3_bond_length.jpg|link=https://en.wikipedia.org/wiki/File:FXeNO3_bond_length.jpg|thumb|Bond length of FXeNO<sub>3</sub>]] |
|||
[[File:FXeNO3_bond_angle.jpg|link=https://en.wikipedia.org/wiki/File:FXeNO3_bond_angle.jpg|thumb|Bond angle of FXeNO<sub>3</sub>]] |
|||
| ⚫ | The bond lengths in the molecule are 1.992 Å for Xe–F, 2.126 Å for Xe–O, 1.36 Å for O–NO<sub>2</sub>, 1.199 for N–O<sub>cis</sub> and 1.224 Å for N–O<sub>trans</sub>.<ref name="MoranM2007" /> The bond angles are 177.6° for F–Xe–O, 114.7° for Xe-O-N, 114.5° for (Xe)O–N–O<sub>cis</sub>, 118.4° for (Xe)O–N–O<sub>trans</sub> and 127.1° for O<sub>cis</sub>–N–O<sub>trans</sub>.<ref name="MoranM2007" /> The bond lengths and angles on the xenon atom are similar to that in FXeOSO<sub>2</sub>F and FXeOTeF<sub>5</sub>, indicating a polar oxygen bond. The Xe–O–N angle is larger than those in halogen nitrates, which indicates a lower bond density for the Xe–O bond.The N–O<sub>cis</sub> bond length is longer than the N–O<sub>trans</sub> bond length, opposite to other halogen nitrates.<ref name="MoranM2007" /> |
||
FXeONO<sub>2</sub> is not particularly stable and slowly breaks down at -78 °C, yielding XeF<sub>2</sub>·N<sub>2</sub>O<sub>4</sub>. This happens on a timescale of several days.<ref name="MoranM2007" /> At 0 °C, FXeONO<sub>2</sub> has a half life of seven hours, decomposing to XeF<sub>2</sub>.<ref name="MoranM2007" /> |
FXeONO<sub>2</sub> is not particularly stable and slowly breaks down at -78 °C, yielding XeF<sub>2</sub>·N<sub>2</sub>O<sub>4</sub>. This happens on a timescale of several days.<ref name="MoranM2007" /> At 0 °C, FXeONO<sub>2</sub> has a half life of seven hours, decomposing to XeF<sub>2</sub>.<ref name="MoranM2007" /> |
||
| Line 29: | Line 58: | ||
{{reflist}} |
{{reflist}} |
||
{{Xenon compounds}} |
|||
{{Noble gas compounds}} |
|||
{{Nitrates}} |
|||
[[Category:Xenon(II) compounds]] |
|||
[[Category:Nitrates]] |
[[Category:Nitrates]] |
||
[[Category:Fluorides]] |
|||
Latest revision as of 20:37, 4 January 2024
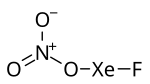
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| FNO3Xe | |
| Molar mass | 212.295 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Xenon fluoride nitrate, also known as fluoroxenonium nitrate, is the chemical compound with formula FXeONO2.[1][2]
Synthesis
[edit]This compound is formed via the reaction:[3]
- [FXeOXeFXeF][AsF6] + 2NO2F → FXeONO2 + NO2AsF6.
Purification of FXeONO2 can then take place by dissolving in SO2ClF, which leaves the nitronium arsenic hexafluoride behind as a solid.[3]
An alternate low yield method to make FXeONO2 is to dissolve xenon difluoride in liquid dinitrogen tetroxide at 0 °C.[3]
- XeF2 + NO+ + NO3− → FXeONO2 + NOF
This method is inefficient as not much nitrate ion exists in the liquid and the xenon fluoride nitrate decomposes.[3]
Another method claimed to make this substance is:[3]
- XeF2 + HNO3 → FXeONO2 + HF
Properties
[edit]FXeONO2 is a white crystalline material.[3] The space group of the crystals is P21/c, which is monoclinic. The unit cell contains four molecules with a total volume of 386.6 Å3. The unit cell dimensions are a = 4.6663 Å, b = 8.799 Å c = 9.415 Å, with non-perpendicular angle β = 90.325°.[3] With a molecular weight of 212.3, the crystal has density 3.648. (These measurements at -173 °C.)[3] The bond lengths in the molecule are 1.992 Å for Xe–F, 2.126 Å for Xe–O, 1.36 Å for O–NO2, 1.199 for N–Ocis and 1.224 Å for N–Otrans.[3] The bond angles are 177.6° for F–Xe–O, 114.7° for Xe-O-N, 114.5° for (Xe)O–N–Ocis, 118.4° for (Xe)O–N–Otrans and 127.1° for Ocis–N–Otrans.[3] The bond lengths and angles on the xenon atom are similar to that in FXeOSO2F and FXeOTeF5, indicating a polar oxygen bond. The Xe–O–N angle is larger than those in halogen nitrates, which indicates a lower bond density for the Xe–O bond. The N–Ocis bond length is longer than the N–Otrans bond length, opposite to other halogen nitrates.[3]
FXeONO2 is not particularly stable and slowly breaks down at -78 °C, yielding XeF2·N2O4. This happens on a timescale of several days.[3] At 0 °C, FXeONO2 has a half life of seven hours, decomposing to XeF2.[3]
References
[edit]- ^ Moran, Matthew D.; David S. Brock; Hélène P. A. Mercier; Gary J. Schrobilgen (2010). "Xe3OF3+, a Precursor to a Noble-Gas Nitrate; Syntheses and Structural Characterizations of FXeONO2, XeF2·HNO3, and XeF2·N2O4". Journal of the American Chemical Society. 132 (39): 13823–13839. doi:10.1021/ja105618w. ISSN 0002-7863. PMID 20843046.
- ^ Atta-ur-Rahman (2006-01-01). Advances in Organic Synthesis: Modern Organofluorine Chemistry-Synthetic Aspects. Bentham Science Publishers. p. 78. ISBN 9781608051984. Retrieved 5 October 2014.
- ^ a b c d e f g h i j k l m Moran, Matthew D. (2007). Synthesis and Structural Characterization of new Xenon(II) Compounds and the Use of a Xenon(II) Cation as an Oxidant for the Preparation of Halogenated Hydrocarbons (PDF). McMaster University. pp. 42, 99–145. Retrieved 4 Oct 2014.
