1,2,3-Benzothiadiazole: Difference between revisions
No edit user (talk | contribs) No edit summary Tags: Visual edit Mobile edit Mobile web edit |
Added publisher. |
||
| (32 intermediate revisions by 13 users not shown) | |||
| Line 1: | Line 1: | ||
{{short description|Organic chemical}} |
{{short description|Organic heterocyclic aromatic chemical}} |
||
<!-- Note: The following pages were redirects to [[1,2,3-Benzothiadiazole]] before draftification: |
|||
| ⚫ | |||
--> |
|||
{{Chembox |
{{Chembox |
||
| Name = 1,2,3-Benzothiadiazole |
| Name = 1,2,3-Benzothiadiazole |
||
| ImageFile = 1,2,3-Benzothiadiazole.png |
| ImageFile = 1,2,3-Benzothiadiazole.png |
||
| OtherNames = {{Unbulleted list|Benzothiadiazole|Benzthiadiazole|Azabenzothiadiazole|benzo-1,2,3-thiadiazole}} |
| OtherNames = {{Unbulleted list|Benzothiadiazole|Benzthiadiazole|Azabenzothiadiazole|benzo-1,2,3-thiadiazole}} |
||
| |
| PIN = 1,2,3-Benzothiadiazole |
||
| ⚫ | |||
| Section1 = {{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| PubChem = 67505 |
| PubChem = 67505 |
||
| CASNo = 273-77-8 |
| CASNo = 273-77-8 |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = R3PA3EL3RP |
|||
| EC_number = 205-989-4 |
| EC_number = 205-989-4 |
||
| MeSHName = benzo-1,2,3-thiadiazole |
| MeSHName = benzo-1,2,3-thiadiazole |
||
| Line 21: | Line 19: | ||
| Section2 ={{chembox Properties |
| Section2 ={{chembox Properties |
||
| C=6|H=4|N=2|S=1 |
| C=6|H=4|N=2|S=1 |
||
| MeltingPtC = 36-37 |
|||
| BoilingPtC = 220.5 |
|||
| Density = 1.499 g/cm<sup>3</sup><ref name=Keith/> |
|||
| Appearance = colorless solid |
|||
}} |
}} |
||
| Section3 = |
| Section3 = |
||
| Section4 = |
| Section4 = |
||
| Section5 = |
| Section5 = {{Chembox Related |
||
| ⚫ | |||
}} |
|||
| Section6 = |
| Section6 = |
||
}} |
}} |
||
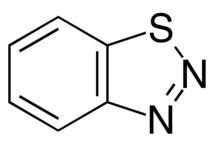
'''1,2,3-Benzothiadiazole''' is a [[bicyclic molecule|bicyclic]] [[Aromaticity|aromatic chemical]] composed of a [[benzene]] ring that is [[fused-ring compound|fused]] to a [[thiadiazoles|1,2,3-thiadiazole]]. A colorless solid, the compound is soluble in organic solvents. |
|||
'''1,2,3-Benzothiadiazole''' is an aromatic heterocyclic<ref>{{Cite journal|title=Search Results - Access Structures|url=https://doi.org/10.5517/cc9618s|language=en|doi=10.5517/cc9618s}}</ref> organic compound which belongs to the benzothiadiazole category. It has a chemical formula of C<sub>6</sub>H<sub>4</sub>N<sub>2</sub>S. |
|||
== Preparation == |
|||
It is a part of the compound [[acibenzolar-S-methyl]], a [[pesticide]].<ref name=":2">{{Cite patent|title=Pesticidal compositions comprising 1,2,3-benzothiadiazole derivatives|gdate=1999-07-28|url=https://patents.google.com/patent/WO2000005959A1/en}}</ref> |
|||
1,2,3-Benzothiadiazole is readily prepared by the [[diazotisation]] reaction of [[2-Aminothiophenol|2-aminothiophenol]] or its [[disulfide]] with [[sodium nitrite]], as originally reported in 1887<ref>{{cite journal |doi=10.1002/cber.188702001423 |title=Zur Kenntniss der orthoamidirten aromatischen Mercaptane |last1=Jacobson |first1=J. |journal=Berichte der Deutschen Chemischen Gesellschaft |year=1887 |volume=20 |pages=1895–1903 }}</ref> and reviewed in several subsequent publications.<ref name=Hodgson>{{cite journal |doi=10.1111/j.1478-4408.1948.tb02498.x |title=A Review of the Chemistry of the Arylthiadiazoles or Internal Diazo–Sulphides |last1=Hodgson |first1=H.H. |last2=Dodoson |first2=D.P. |journal=Journal of the Society of Dyers and Colourists |year=1964 |volume=64 |issue=2 |pages=65–71 }}</ref><ref>{{cite book |doi=10.1016/B978-008096519-2.00092-8 |chapter=1,2,3-Thiadiazoles and their Benzo Derivatives |title=Comprehensive Heterocyclic Chemistry |year=1984 |last1=Thomas |first1=E.W. |pages=447–462 |isbn=9780080965192 }}</ref><ref>{{cite book |title= Science of Synthesis |volume= 13: Category 2, Hetarenes and Related Ring Systems |editor1-last= Storr |editor1-first= R. C. |editor2-last= Gilchrist |editor2-first= T. L. |year= 2004 |doi= 10.1055/sos-SD-013-00386 |chapter= Product Class 9: 1,2,3-Thiadiazoles|isbn= 9783131122810 }}</ref><ref>{{cite book |isbn=9783131812445 |title=Houben-Weyl Methods of Organic Chemistry Vol. E 8d, 4th Edition Supplement: Hetarenes III (Five-Membered Rings with Two and More Heteroatoms in the Ring System) - Part 4 |date=14 May 2014 |pages=93–104 |chapter=1,2,3-Benzothiadiazole |publisher=Georg Thieme Verlag }}</ref> |
|||
| ⚫ | |||
By the [[Herz reaction]] [[aniline]]s can be converted to benzothiadiazole. The method is attractive since less elaborate precursors (merely anilines) are required. Upon treatment with [[disulfur dichloride]], the anilines give the intermediate 1,3,2-benzothiazathiolium salt, which is diazotised to complete the formation of a 1,2,3-benzothiadiazole. The parent system cannot be made this way, since the use of aniline in this reaction leads to formation of the 6-chloro derivative.<ref name=Kirby/> |
|||
==Structure and bonding== |
|||
The molecule is planar. The N-N and S-N distances are respectively 128 and 171 [[picometer]]s, indicative of multiple bond character.<ref name=Keith>{{cite journal |doi=10.1016/0022-328X(91)86290-7 |title=Transition metal heterocyclic chemistry: XI. Manganese cyclopentadienyldicarbonyl complexes of 1,2,3-selena- and thiadiazoles including structural comparison of free and complexed 1,2,3-benzothiadiazole and 4-phenyl-1,2,3-thiadiazole |year=1991 |last1=Mayr |first1=Armin J. |last2=Carrasco-Flores |first2=Benjamin |last3=Cervantes-Lee |first3=Francisco |last4=Pannell |first4=Keith H. |last5=Párkányi |first5=László |last6=Raghuveer |first6=Krishan |journal=Journal of Organometallic Chemistry |volume=405 |issue=3 |pages=309–322 }} |
|||
</ref> Like [[naphthalene]], this heterocycle is a 10-electron system.<ref name=Hodgson /> |
|||
| ⚫ | |||
1,2,3-benzothiadiazole is much less nucleophilic than naphthalene. [[Nitration]] is slow.<ref>{{cite journal |doi=10.1039/JR9630004794 |title=1,2,3-Benzothiadiazole. Part II. Electrophilic substitution in 4- and 6-amino-1,2,3-benzothiadiazoles |year=1963 |last1=Ward |first1=E. R. |last2=Heard |first2=D. D. |journal=Journal of the Chemical Society (Resumed) |pages=4794–4803 }}</ref> For that reason, many of its simple derivatives have been made from 2-aminothiophenols already having additional substituents.<ref name=Kirby>{{cite journal |doi=10.1039/J39700002250 |title=1,2,3-Benzothiadiazoles. Part I. A simplified synthesis of 1,2,3-benzothiadiazoles |year=1970 |last1=Kirby |first1=P. |last2=Soloway |first2=S. B. |last3=Davies |first3=J. H. |last4=Webb |first4=Shirley B. |journal=Journal of the Chemical Society C: Organic |issue=16 |page=2250 }}</ref> |
|||
1,2,3-benzothiadiazole is a very weak base and [[alkylation]] reactions give exclusively the 3-amino [[Quaternary ammonium cation|quaternary salt]].<ref>{{cite journal |doi=10.1039/J39700002060 |title=1,2,3-Benzothiadiazole. Part VI. Investigations on the quaternisation of 1,2,3-benzothiadiazole and 1,2,3-benzoselenadiazole |year=1970 |last1=Jaffari |first1=G. A. |last2=Nunn |first2=A. J. |last3=Ralph |first3=J. T. |journal=Journal of the Chemical Society C: Organic |issue=15 |page=2060 }}</ref> |
|||
== Properties == |
|||
== Applications == |
|||
1,2,3-Benzothiadiazole has a chemical formula of C<sub>6</sub>H<sub>4</sub>N<sub>2</sub>S. |
|||
1,2,3-benzothiadiazole has been claimed to [[Synergy#Biological_sciences|synergise]] [[insecticides]] including [[dicrotophos]]<ref>{{cite journal |doi=10.1021/jf60170a011 |title=Benzothiadiazoles, a novel group of insecticide synergists |year=1970 |last1=Felton |first1=John C. |last2=Jenner |first2=Donald W. |last3=Kirby |first3=Peter. |journal=Journal of Agricultural and Food Chemistry |volume=18 |issue=4 |pages=671–673 }}</ref> but has not been commercialised for that application. The only derivative to have found significant use is the [[fungicide]] [[acibenzolar-S-methyl|acibenzolar-''S''-methyl]]. |
|||
| ⚫ | |||
1,2,3-Benzothiadiazole reacts with [[Fluorine]],[[Chlorine]],[[Bromine]] and [[Iodine]] to form their own respective 1,2,3-Benzothiadiazoles.<ref name=":1">{{Cite journal|last2=Poesche|first2=W. H.|last3=Higgins|first3=D.|last4=Heard|first4=D. D.|title=458. 1,2,3-Benzothiadiazole. Part I. Nitro-, amino-, and hydroxy-derivatives|url=https://pubs.rsc.org/en/content/articlelanding/1962/jr/jr9620002374|journal=Journal of the Chemical Society (Resumed)|year=1962|language=en|volume=|pages=2374–2379|doi=10.1039/JR9620002374|issn=0368-1769|via=|last1=Ward|first1=E. R.}}</ref>It may also react with [[Nitrogen]] and [[Amine]] to create their own respective 1,2,3-Benzothiadiazoles.<ref name=":1" />The fluoro-,chloro-,bromo- and iodo- 1,2,3-Benzothiadiazoles are used / found in electrophilic solutions.<ref>{{Cite journal|last1=Ward|first1=E. R.|last2=Heard|first2=D. D.|date=1965-01-01|title=183. 1,2,3-Benzothiadiazole. Part III. Electrophilic substitution in 5- and 7-amino-1,2,3-benzothiadiazoles and the preparation of some substituted 1,2,3-benzothiadiazoles|url=https://pubs.rsc.org/en/content/articlelanding/1965/jr/jr9650001023|journal=Journal of the Chemical Society (Resumed)|language=en|pages=1023–1028|doi=10.1039/JR9650001023|issn=0368-1769}}</ref> |
|||
Some of 1,2,3-Benzothiadiazole derivatives are either [[Pesticides]] or [[Herbicides]].<ref name=":2" /><ref>{{Cite web|last=Google|first=Patents|date=|title=US3536728A-1,2,3-Benzothiadiazole|url=https://www.patents.google.com/patents/US3536728A/en|url-status=live|archive-url=|archive-date=|access-date=|website=}}</ref>1,2,3-Benzothiadiazole has many more reactions and their derivatives, some of the most common ones being [[1,2,3-Benzothiadiazole-7-carboxylic acid]] and [[Acibenzolar-S-methyl]]. |
|||
==Production. == |
|||
1,2,3-Benzothiadiazole is readily prepared by the [[diazotisation]] reaction of [[2-Aminothiophenol|2-aminothiophenol]] with [[sodium nitrite]]. The [[Chemistry]] of this [[heterocycle]] and its simple derivatives have been reviewed.<ref>{{cite book |isbn=9783131812445 |title=Houben-Weyl Methods of Organic Chemistry Vol. E 8d, 4th Edition Supplement: Hetarenes III (Five-Membered Rings with Two and More Heteroatoms in the Ring System) - Part 4 |date=14 May 2014 |pages=93–104 |chapter=1,2,3-Benzothiadiazole }}</ref> |
|||
== History == |
|||
1,2,3-Benzothiadiazole has been known since 1888.<ref>{{cite book |title= Science of Synthesis |volume= 13: Category 2, Hetarenes and Related Ring Systems |editor1-last= Storr |editor1-first= R. C. |editor2-last= Gilchrist |editor2-first= T. L. |year= 2004 |doi= 10.1055/sos-SD-013-00386 |chapter= Product Class 9: 1,2,3-Thiadiazoles|isbn= 9783131122810 }}</ref><ref name=":0">{{Cite web|last=|first=|date=|title=1,2,3-Thiadiazoles and their Benzo derivatives|url=https://www.sciencedirect.com/science/article/pii/B9780080965192000928|url-status=live|archive-url=|archive-date=|access-date=|website=}}</ref> |
|||
== References == |
== References == |
||
<!-- Inline citations added to your article will automatically display here. See en.wikipedia.org/wiki/WP:REFB for instructions on how to add citations. --> |
|||
{{reflist}} |
{{reflist}} |
||
| ⚫ | |||
[[Category:Nitrogen heterocycles]] |
|||
| ⚫ | |||
[[Category:Nitrogen heterocycles]] |
|||
[[Category:Heterocyclic compounds (2 rings)]] |
|||
Latest revision as of 09:39, 11 January 2024
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,2,3-Benzothiadiazole | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| MeSH | benzo-1,2,3-thiadiazole |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H4N2S | |
| Molar mass | 136.17 g·mol−1 |
| Appearance | colorless solid |
| Density | 1.499 g/cm3[1] |
| Melting point | 36–37 °C (97–99 °F; 309–310 K) |
| Boiling point | 220.5 °C (428.9 °F; 493.6 K) |
| Related compounds | |
Related compounds
|
2,1,3-Benzothiadiazole |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1,2,3-Benzothiadiazole is a bicyclic aromatic chemical composed of a benzene ring that is fused to a 1,2,3-thiadiazole. A colorless solid, the compound is soluble in organic solvents.
Preparation
[edit]1,2,3-Benzothiadiazole is readily prepared by the diazotisation reaction of 2-aminothiophenol or its disulfide with sodium nitrite, as originally reported in 1887[2] and reviewed in several subsequent publications.[3][4][5][6]
By the Herz reaction anilines can be converted to benzothiadiazole. The method is attractive since less elaborate precursors (merely anilines) are required. Upon treatment with disulfur dichloride, the anilines give the intermediate 1,3,2-benzothiazathiolium salt, which is diazotised to complete the formation of a 1,2,3-benzothiadiazole. The parent system cannot be made this way, since the use of aniline in this reaction leads to formation of the 6-chloro derivative.[7]
Structure and bonding
[edit]The molecule is planar. The N-N and S-N distances are respectively 128 and 171 picometers, indicative of multiple bond character.[1] Like naphthalene, this heterocycle is a 10-electron system.[3]
Reactions
[edit]1,2,3-benzothiadiazole is much less nucleophilic than naphthalene. Nitration is slow.[8] For that reason, many of its simple derivatives have been made from 2-aminothiophenols already having additional substituents.[7]
1,2,3-benzothiadiazole is a very weak base and alkylation reactions give exclusively the 3-amino quaternary salt.[9]
Applications
[edit]1,2,3-benzothiadiazole has been claimed to synergise insecticides including dicrotophos[10] but has not been commercialised for that application. The only derivative to have found significant use is the fungicide acibenzolar-S-methyl.
References
[edit]- ^ a b Mayr, Armin J.; Carrasco-Flores, Benjamin; Cervantes-Lee, Francisco; Pannell, Keith H.; Párkányi, László; Raghuveer, Krishan (1991). "Transition metal heterocyclic chemistry: XI. Manganese cyclopentadienyldicarbonyl complexes of 1,2,3-selena- and thiadiazoles including structural comparison of free and complexed 1,2,3-benzothiadiazole and 4-phenyl-1,2,3-thiadiazole". Journal of Organometallic Chemistry. 405 (3): 309–322. doi:10.1016/0022-328X(91)86290-7.
- ^ Jacobson, J. (1887). "Zur Kenntniss der orthoamidirten aromatischen Mercaptane". Berichte der Deutschen Chemischen Gesellschaft. 20: 1895–1903. doi:10.1002/cber.188702001423.
- ^ a b Hodgson, H.H.; Dodoson, D.P. (1964). "A Review of the Chemistry of the Arylthiadiazoles or Internal Diazo–Sulphides". Journal of the Society of Dyers and Colourists. 64 (2): 65–71. doi:10.1111/j.1478-4408.1948.tb02498.x.
- ^ Thomas, E.W. (1984). "1,2,3-Thiadiazoles and their Benzo Derivatives". Comprehensive Heterocyclic Chemistry. pp. 447–462. doi:10.1016/B978-008096519-2.00092-8. ISBN 9780080965192.
- ^ Storr, R. C.; Gilchrist, T. L., eds. (2004). "Product Class 9: 1,2,3-Thiadiazoles". Science of Synthesis. Vol. 13: Category 2, Hetarenes and Related Ring Systems. doi:10.1055/sos-SD-013-00386. ISBN 9783131122810.
- ^ "1,2,3-Benzothiadiazole". Houben-Weyl Methods of Organic Chemistry Vol. E 8d, 4th Edition Supplement: Hetarenes III (Five-Membered Rings with Two and More Heteroatoms in the Ring System) - Part 4. Georg Thieme Verlag. 14 May 2014. pp. 93–104. ISBN 9783131812445.
- ^ a b Kirby, P.; Soloway, S. B.; Davies, J. H.; Webb, Shirley B. (1970). "1,2,3-Benzothiadiazoles. Part I. A simplified synthesis of 1,2,3-benzothiadiazoles". Journal of the Chemical Society C: Organic (16): 2250. doi:10.1039/J39700002250.
- ^ Ward, E. R.; Heard, D. D. (1963). "1,2,3-Benzothiadiazole. Part II. Electrophilic substitution in 4- and 6-amino-1,2,3-benzothiadiazoles". Journal of the Chemical Society (Resumed): 4794–4803. doi:10.1039/JR9630004794.
- ^ Jaffari, G. A.; Nunn, A. J.; Ralph, J. T. (1970). "1,2,3-Benzothiadiazole. Part VI. Investigations on the quaternisation of 1,2,3-benzothiadiazole and 1,2,3-benzoselenadiazole". Journal of the Chemical Society C: Organic (15): 2060. doi:10.1039/J39700002060.
- ^ Felton, John C.; Jenner, Donald W.; Kirby, Peter. (1970). "Benzothiadiazoles, a novel group of insecticide synergists". Journal of Agricultural and Food Chemistry. 18 (4): 671–673. doi:10.1021/jf60170a011.

