2-Ethylanthraquinone: Difference between revisions
typo correction |
Added isbn. |
||
| (14 intermediate revisions by 13 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Intermediate Chemical in H2O2 Synthesis}} |
|||
{{chembox |
|||
{{Chembox |
|||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| verifiedrevid = 477213314 |
| verifiedrevid = 477213314 |
||
| Line 9: | Line 10: | ||
| ImageName1 = Ball-and-stick model |
| ImageName1 = Ball-and-stick model |
||
| ImageSize1 = 230px |
| ImageSize1 = 230px |
||
| PIN = 2-Ethylanthracene-9,10-dione |
|||
| OtherNames = 2-Ethyl-9,10-anthracenedione |
| OtherNames = 2-Ethyl-9,10-anthracenedione |
||
| Section1 = {{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
||
| ChemSpiderID = 6514 |
| ChemSpiderID = 6514 |
||
| PubChem = 6772 |
|||
| UNII = 59YJ81QZKD |
|||
| InChI = 1/C16H12O2/c1-2-10-7-8-13-14(9-10)16(18)12-6-4-3-5-11(12)15(13)17/h3-9H,2H2,1H3 |
| InChI = 1/C16H12O2/c1-2-10-7-8-13-14(9-10)16(18)12-6-4-3-5-11(12)15(13)17/h3-9H,2H2,1H3 |
||
| InChIKey = SJEBAWHUJDUKQK-UHFFFAOYAW |
| InChIKey = SJEBAWHUJDUKQK-UHFFFAOYAW |
||
| Line 35: | Line 39: | ||
}} |
}} |
||
| Section7 = {{Chembox Hazards |
| Section7 = {{Chembox Hazards |
||
| ⚫ | |||
| SPhrases = {{S24}} {{S25}} |
|||
| GHSPictograms = {{GHS08}}{{GHS09}} |
|||
| ⚫ | |||
| GHSSignalWord = Danger |
|||
| HPhrases = {{H-phrases|350|373|410}} |
|||
| PPhrases = {{P-phrases|201|202|260|273|281|308+313|314|391|405|501}} |
|||
}} |
}} |
||
}} |
}} |
||
'''2-Ethylanthraquinone''' is an [[organic compound]] that is a derivative of [[anthraquinone]]. |
'''2-Ethylanthraquinone''' is an [[organic compound]] that is a derivative of [[anthraquinone]]. This pale yellow solid is used in the industrial production of [[hydrogen peroxide]] (H<sub>2</sub>O<sub>2</sub>).<ref>{{cite encyclopedia |author1=Goor, G. |author2=Glenneberg, J. |author3=Jacobi, S. | title = Hydrogen Peroxide | encyclopedia = Ullmann's Encyclopedia of Industrial Chemistry | year = 2007 | publisher = Wiley-VCH | location = Weinheim | doi = 10.1002/14356007.a13_443.pub2 |isbn=9783527303854 }}</ref><ref>Römpp CD 2006, Georg Thieme Verlag 2006</ref> |
||
==Production== |
==Production== |
||
| Line 48: | Line 55: | ||
==Uses== |
==Uses== |
||
[[Hydrogen peroxide]] is produced industrially by the [[anthraquinone process]] which involves using 2-alkyl-9,10-anthraquinones for hydrogenation. Many derivatives of anthraquinone are used but 2-ethylanthraquinone is common because of its high selectivity. The hydrogenation of the |
[[Hydrogen peroxide]] is produced industrially by the [[anthraquinone process]] which involves using 2-alkyl-9,10-anthraquinones for hydrogenation. Many derivatives of anthraquinone are used but 2-ethylanthraquinone is common because of its high selectivity. The hydrogenation of the unsubstituted ring can reach 90% selectivity by using 2-ethylanthraquinone. Hydrogenation follows the Riedl-Pfleiderer, or [[autoxidation]], process: |
||
[[File:Riedl-Pfleiderer process.svg|left|400px|The Riedl-Pfleiderer process.]] |
[[File:Riedl-Pfleiderer process.svg|left|400px|The Riedl-Pfleiderer process.]]{{clear|left}} |
||
The [[hydrogenation]] of [[2-ethylanthraquinone]] is catalyzed by [[palladium]]. Hydrogenation produces both 2-ethylanthrahydroquinone and tetrahydroanthraquinone. The tetrahydro derivative of 2-alkylanthraquinone is easily |
The [[hydrogenation]] of [[2-ethylanthraquinone]] is catalyzed by [[palladium]]. Hydrogenation produces both 2-ethylanthrahydroquinone and tetrahydroanthraquinone. The tetrahydro derivative of 2-alkylanthraquinone is easily hydrogenated but is more difficult to oxidize. The formation of the tetrahydro derivative can be suppressed through the selection of catalysts, solvents, and reaction conditions. Some suggested solvent mixtures are polyalkylated benzenes and alkyl phosphates or tetraalkyl ureas, trimethylbenzenes and alkylcyclohexanol esters, and methylnaphthalene and nonyl alcohols. |
||
==References== |
==References== |
||
Latest revision as of 10:04, 13 January 2024
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Ethylanthracene-9,10-dione | |
| Other names
2-Ethyl-9,10-anthracenedione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.396 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H12O2 | |
| Molar mass | 236.27 g/mol |
| Appearance | white to yellowish crystals or powder |
| Density | 1.231g/cm3 |
| Melting point | 105 °C (221 °F; 378 K) |
| Boiling point | 415.4 @ 760mmHg |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H350, H373, H410 | |
| P201, P202, P260, P273, P281, P308+P313, P314, P391, P405, P501 | |
| Flash point | 155.4 °C (311.7 °F; 428.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Ethylanthraquinone is an organic compound that is a derivative of anthraquinone. This pale yellow solid is used in the industrial production of hydrogen peroxide (H2O2).[1][2]
Production
[edit]2-Ethylanthraquinone is prepared from the reaction of phthalic anhydride and ethylbenzene:
- C6H4(CO)2O + C6H5Et → C6H4(CO)2C6H3Et + H2O.
Both phthalic anhydride and ethylbenzene are readily available, being otherwise used in the large-scale production of plastics.
Uses
[edit]Hydrogen peroxide is produced industrially by the anthraquinone process which involves using 2-alkyl-9,10-anthraquinones for hydrogenation. Many derivatives of anthraquinone are used but 2-ethylanthraquinone is common because of its high selectivity. The hydrogenation of the unsubstituted ring can reach 90% selectivity by using 2-ethylanthraquinone. Hydrogenation follows the Riedl-Pfleiderer, or autoxidation, process:
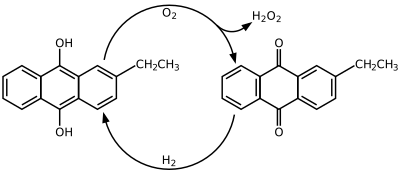
The hydrogenation of 2-ethylanthraquinone is catalyzed by palladium. Hydrogenation produces both 2-ethylanthrahydroquinone and tetrahydroanthraquinone. The tetrahydro derivative of 2-alkylanthraquinone is easily hydrogenated but is more difficult to oxidize. The formation of the tetrahydro derivative can be suppressed through the selection of catalysts, solvents, and reaction conditions. Some suggested solvent mixtures are polyalkylated benzenes and alkyl phosphates or tetraalkyl ureas, trimethylbenzenes and alkylcyclohexanol esters, and methylnaphthalene and nonyl alcohols.
References
[edit]- ^ Goor, G.; Glenneberg, J.; Jacobi, S. (2007). "Hydrogen Peroxide". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_443.pub2. ISBN 9783527303854.
- ^ Römpp CD 2006, Georg Thieme Verlag 2006
