2-Ethoxyethanol: Difference between revisions
Appearance
Content deleted Content added
Updating {{chembox}} (changes to verified fields - updated 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chemicals| |
Cellosolve is not specific to just this glycol ether |
||
| (42 intermediate revisions by 26 users not shown) | |||
| Line 1: | Line 1: | ||
{{chembox |
{{chembox |
||
| |
|Verifiedfields = changed |
||
|Watchedfields = changed |
|||
| verifiedrevid = 387278787 |
|||
|verifiedrevid = 477213258 |
|||
| Name = 2-Ethoxyethanol |
|||
| |
|Name = 2-Ethoxyethanol |
||
|ImageFile = 2-Ethoxyethanol2.svg |
|||
|ImageName = 2-Ethoxyethanol |
|||
| ImageSize = 200px |
|||
| |
|PIN = 2-Ethoxyethanol |
||
|OtherNames = Cellosolve<br />ethylene glycol ethyl ether<br />oxitol<br />Ethyl Cellosolve<br/>EGEE |
|||
| IUPACName = 2-ethoxyethanol |
|||
|Section1={{Chembox Identifiers |
|||
| OtherNames = Cellosolve<br />ethylene glycol ethyl ether<br />oxitol<br />Ethyl Cellosolve |
|||
|DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| Section1 = {{Chembox Identifiers |
|||
|DrugBank = DB02249 |
|||
| SMILES = CCOCCO |
|||
| |
|ChEBI_Ref = {{ebicite|correct|EBI}} |
||
|ChEBI = 46788 |
|||
| ChemSpiderID = 7785 |
|||
| |
|KEGG_Ref = {{keggcite|correct|kegg}} |
||
| |
|KEGG = C14687 |
||
|PubChem = 8076 |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
|ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| UNII = IDK7C2HS09 |
|||
|ChemSpiderID = 13836591 |
|||
| CASNo_Ref = {{cascite}} |
|||
| |
|EC_number = 203-804-1 |
||
|UNNumber = 1171 |
|||
| RTECS = KK8050000 |
|||
|Beilstein = 1098271 |
|||
}} |
|||
|Gmelin = 82142 |
|||
| Section2 = {{Chembox Properties |
|||
|SMILES = CCOCCO |
|||
| C=4|H=10|O=2 |
|||
|InChI = 1/C4H10O2/c1-2-6-4-3-5/h5H,2-4H2,1H3 |
|||
| Appearance = clear liquid |
|||
|InChIKey = ZNQVEEAIQZEUHB-UHFFFAOYAD |
|||
| Density = 0.930 g/cm<sup>3</sup>, liquid |
|||
|StdInChI = 1S/C4H10O2/c1-2-6-4-3-5/h5H,2-4H2,1H3 |
|||
| Solubility = miscible |
|||
|StdInChIKey = ZNQVEEAIQZEUHB-UHFFFAOYSA-N |
|||
| MeltingPtC = -70 |
|||
|ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
| BoilingPtC = 135 |
|||
|ChEMBL = 119596 |
|||
| Viscosity = |
|||
|UNII_Ref = {{fdacite|correct|FDA}} |
|||
}} |
|||
|UNII = IDK7C2HS09 |
|||
| Section7 = {{Chembox Hazards |
|||
|CASNo_Ref = {{cascite|correct|CAS}} |
|||
| ExternalMSDS = |
|||
|CASNo = 110-80-5 |
|||
| MainHazards = |
|||
|RTECS = KK8050000 |
|||
| NFPA-H = 2 |
|||
}} |
|||
| NFPA-F = 2 |
|||
|Section2={{Chembox Properties |
|||
| NFPA-R = |
|||
| |
|C=4 | H=10 | O=2 |
||
|Appearance = clear liquid |
|||
| RPhrases = {{R10}}, {{R20/21/22}},<br />{{R60}}, {{R61}} |
|||
|Odor = sweet, [[ether]]-like |
|||
| SPhrases = {{S53}}, {{S45}} |
|||
|Density = 0.930 g/cm<sup>3</sup>, liquid |
|||
}} |
|||
|Solubility = miscible |
|||
| Section8 = {{Chembox Related |
|||
|MeltingPtC = -70 |
|||
| Function = [[ether]]s |
|||
|BoilingPtC = 135 |
|||
| OtherFunctn = [[2-Propoxyethanol]]<br />[[2-Butoxyethanol]] |
|||
|VaporPressure = 4 mmHg (20°C)<ref name=PGCH/> |
|||
| OtherCpds = [[Ethylene glycol]] |
|||
}} |
|||
|Section7={{Chembox Hazards |
|||
|NFPA-H = 2 |
|||
|NFPA-F = 2 |
|||
|NFPA-R = |
|||
|FlashPtC = 44 |
|||
|GHSPictograms = {{GHS02}}{{GHS06}}{{GHS07}}{{GHS08}} |
|||
|GHSSignalWord = Danger |
|||
|HPhrases = {{H-phrases|226|302|331|360}} |
|||
|PPhrases = {{P-phrases|201|202|210|233|240|241|242|243|261|264|270|271|280|281|301+312|303+361+353|304+340|308+313|311|321|330|370+378|403+233|403+235|405|501}} |
|||
|PEL = TWA 200 ppm (740 mg/m<sup>3</sup>) [skin]<ref name=PGCH>{{PGCH|0258}}</ref> |
|||
|ExploLimits = 1.7%-15.6%<ref name=PGCH/> |
|||
|IDLH = 500 ppm<ref name=PGCH/> |
|||
|REL = TWA 0.5 ppm (1.8 mg/m<sup>3</sup>) [skin]<ref name=PGCH/> |
|||
|LC50 = 2000 ppm (rat, 7 hr)<br/>1820 ppm (mouse, 7 hr)<ref name=IDLH>{{IDLH|110805|2-Ethoxyethanol}}</ref> |
|||
|LD50 = 2451 mg/kg (mouse, oral)<br/>2125 mg/kg (rat, oral)<ref name=IDLH/> |
|||
|LCLo = 3000 ppm (guinea pig, 24 hr)<ref name=IDLH/> |
|||
}} |
|||
|Section8={{Chembox Related |
|||
|OtherFunction_label = [[ether]]s |
|||
|OtherFunction = [[2-Propoxyethanol]]<br />[[2-Butoxyethanol]] |
|||
|OtherCompounds = [[Ethylene glycol]] |
|||
}} |
|||
}} |
}} |
||
'''2-Ethoxyethanol''', also known by the trademark ''' |
'''2-Ethoxyethanol''', also known by the trademark '''Ethyl cellosolve''', is a [[solvent]] used widely in commercial and industrial applications. It is a clear, colorless, nearly odorless liquid that is miscible with water, [[ethanol]], [[diethyl ether]], [[acetone]], and [[ethyl acetate]].<ref name="Concentrations1996">{{cite book|author=National Research Council (U.S.). Subcommittee on Spacecraft Maximum Allowable Concentrations|title=Spacecraft maximum allowable concentrations for selected airborne contaminants|url=https://books.google.com/books?id=3VZRtLk--BAC&pg=PT210|access-date=19 February 2012|year=1996|publisher=National Academies Press|isbn=978-0-309-05478-2|page=189}}</ref> |
||
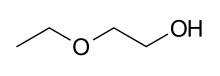
2-Ethoxyethanol |
2-Ethoxyethanol is manufactured by the reaction of [[ethylene oxide]] with [[ethanol]]. |
||
As with other [[glycol ether]]s, 2-ethoxyethanol has the useful property of being able to dissolve chemically diverse compounds. It will dissolve oils, resins, grease, waxes, nitrocellulose, and lacquers. This is an ideal property as a multi-purpose cleaner and therefore 2-ethoxyethanol is used in products such as varnish removers and degreasing solutions. |
As with other [[glycol ether]]s, 2-ethoxyethanol has the useful property of being able to dissolve chemically diverse compounds. It will dissolve oils, resins, grease, waxes, [[nitrocellulose]], and lacquers.<ref name="Concentrations1996"/> This is an ideal property as a multi-purpose cleaner, and, therefore, 2-ethoxyethanol is used in products such as varnish removers and degreasing solutions. |
||
==References== |
==References== |
||
{{Citation style|date=September 2007}} |
|||
<references/> |
<references/> |
||
*''Merck Index'', 11th Edition, '''3707'''. |
|||
==External links== |
==External links== |
||
*{{cite web|title=Chemical Sampling Information 2-Ethoxyethanol|url=https://www.osha.gov/dts/chemicalsampling/data/CH_239200.html|website=www.osha.gov|access-date=4 August 2014|archive-date=8 August 2014|archive-url=https://web.archive.org/web/20140808051237/https://www.osha.gov/dts/chemicalsampling/data/CH_239200.html|url-status=dead}} |
|||
*[http://www.osha.gov/SLTC/healthguidelines/2-ethoxyethanol/recognition.html OSHA guidelines for 2-ethoxyethanol] |
|||
*[https://www.cdc.gov/niosh/npg/npgd0258.html CDC - NIOSH Pocket Guide to Chemical Hazards] |
|||
*[http://elarum.com/info/products/ethylcellosolve/ Brief technical specification of ethylcellosolve] |
|||
{{DEFAULTSORT:Ethoxyethanol, 2-}} |
{{DEFAULTSORT:Ethoxyethanol, 2-}} |
||
[[Category: |
[[Category:Primary alcohols]] |
||
[[Category:Glycol ethers]] |
[[Category:Glycol ethers]] |
||
[[de:2-Ethoxyethanol]] |
|||
[[nl:2-ethoxyethanol]] |
|||
[[pt:2-Etoxietanol]] |
|||
[[ru:Этилцеллозольв]] |
|||
[[zh:乙二醇单乙醚]] |
|||
Latest revision as of 20:56, 12 February 2024
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Ethoxyethanol | |
| Other names
Cellosolve
ethylene glycol ethyl ether oxitol Ethyl Cellosolve EGEE | |
| Identifiers | |
3D model (JSmol)
|
|
| 1098271 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.459 |
| EC Number |
|
| 82142 | |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1171 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H10O2 | |
| Molar mass | 90.122 g·mol−1 |
| Appearance | clear liquid |
| Odor | sweet, ether-like |
| Density | 0.930 g/cm3, liquid |
| Melting point | −70 °C (−94 °F; 203 K) |
| Boiling point | 135 °C (275 °F; 408 K) |
| miscible | |
| Vapor pressure | 4 mmHg (20°C)[1] |
| Hazards | |
| GHS labelling: | |
   
| |
| Danger | |
| H226, H302, H331, H360 | |
| P201, P202, P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P281, P301+P312, P303+P361+P353, P304+P340, P308+P313, P311, P321, P330, P370+P378, P403+P233, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 44 °C (111 °F; 317 K) |
| Explosive limits | 1.7%-15.6%[1] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
2451 mg/kg (mouse, oral) 2125 mg/kg (rat, oral)[2] |
LC50 (median concentration)
|
2000 ppm (rat, 7 hr) 1820 ppm (mouse, 7 hr)[2] |
LCLo (lowest published)
|
3000 ppm (guinea pig, 24 hr)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 200 ppm (740 mg/m3) [skin][1] |
REL (Recommended)
|
TWA 0.5 ppm (1.8 mg/m3) [skin][1] |
IDLH (Immediate danger)
|
500 ppm[1] |
| Related compounds | |
Related ethers
|
2-Propoxyethanol 2-Butoxyethanol |
Related compounds
|
Ethylene glycol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Ethoxyethanol, also known by the trademark Ethyl cellosolve, is a solvent used widely in commercial and industrial applications. It is a clear, colorless, nearly odorless liquid that is miscible with water, ethanol, diethyl ether, acetone, and ethyl acetate.[3]
2-Ethoxyethanol is manufactured by the reaction of ethylene oxide with ethanol.
As with other glycol ethers, 2-ethoxyethanol has the useful property of being able to dissolve chemically diverse compounds. It will dissolve oils, resins, grease, waxes, nitrocellulose, and lacquers.[3] This is an ideal property as a multi-purpose cleaner, and, therefore, 2-ethoxyethanol is used in products such as varnish removers and degreasing solutions.
References
[edit]- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0258". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b c "2-Ethoxyethanol". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ a b National Research Council (U.S.). Subcommittee on Spacecraft Maximum Allowable Concentrations (1996). Spacecraft maximum allowable concentrations for selected airborne contaminants. National Academies Press. p. 189. ISBN 978-0-309-05478-2. Retrieved 19 February 2012.
External links
[edit]- "Chemical Sampling Information 2-Ethoxyethanol". www.osha.gov. Archived from the original on 8 August 2014. Retrieved 4 August 2014.
- CDC - NIOSH Pocket Guide to Chemical Hazards

