Benzene (data page): Difference between revisions
Appearance
Content deleted Content added
refs |
m →Structure and properties: Task 17 - remove UTM parameters (Google analytics) from URLs |
||
| (21 intermediate revisions by 11 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical data page}} |
|||
{{Use dmy dates|date= |
{{Use dmy dates|date=February 2023}} |
||
<!-- To obtain a blank version of this page, type subst:chembox supplement inside of double curly braces, , and save the page --> |
<!-- To obtain a blank version of this page, type subst:chembox supplement inside of double curly braces, , and save the page --> |
||
| Line 6: | Line 7: | ||
== Material Safety Data Sheet == <!-- KEEP this header, it is linked to from the infobox on the main article page --> |
== Material Safety Data Sheet == <!-- KEEP this header, it is linked to from the infobox on the main article page --> |
||
The handling of this chemical may incur notable safety precautions. It is highly |
The handling of this chemical may incur notable safety precautions. It is highly recommended to seek the Material Safety Datasheet ([[Material safety data sheet|MSDS]]) for this chemical from a reliable source such as [https://web.archive.org/web/20070630043133/http://siri.org/msds/index.php SIRI], and follow its directions. MSDS for '''benzene''' is available at [https://web.archive.org/web/20070927234542/http://www2.siri.org/msds/mf/amoco/files/11697000.html AMOCO]. |
||
== Structure and properties == <!-- KEEP this header, it is linked to from the infobox on the main article page --> |
== Structure and properties == <!-- KEEP this header, it is linked to from the infobox on the main article page --> |
||
| Line 13: | Line 14: | ||
! {{chembox header}} | Structure and properties |
! {{chembox header}} | Structure and properties |
||
|- |
|- |
||
| [[ |
| [[Refractive index]], ''n''<sub>D</sub> |
||
| 1.5011 at 20°C <!-- Please omit if not applicable --> |
| 1.5011 at 20 °C <!-- Please omit if not applicable --> |
||
|- |
|- |
||
| [[Abbe number]] |
| [[Abbe number]] |
||
|? <!-- Please omit if not applicable --> |
|? <!-- Please omit if not applicable --> |
||
|- |
|- |
||
| [[Dielectric constant]], ε<sub>r</sub> |
| [[Relative permittivity#Terminology|Dielectric constant]], ε<sub>r</sub> |
||
| (2.274 – 0.0020Δ''T'') ε<sub>0</sub><br>(Δ''T'' = ''T'' – 25 |
| (2.274 – 0.0020Δ''T'') ε<sub>0</sub><br>(Δ''T'' = ''T'' – 25 °C) |
||
|- |
|- |
||
| [[Bond |
| [[Bond energy]] |
||
| ? <!-- Specify which bond. Please omit if not applicable --> |
| ? <!-- Specify which bond. Please omit if not applicable --> |
||
|- |
|- |
||
| [[Bond length]] |
| [[Bond length]] |
||
| 1.39 Å C-C<ref>{{cite book| |
| 1.39 Å C-C<ref>{{cite book|author=Brown|author2=LeMay|author3=Bursten|title=Chemistry: The Central Science|year=2006|publisher=Pearson Education|location=Upper Saddle River, NJ|isbn=0-13-109686-9|pages=[https://archive.org/details/chemistry00theo_0/page/1067 1067]|url-access=registration|url=https://archive.org/details/chemistry00theo_0}}</ref> |
||
|- |
|- |
||
| [[ |
| [[Molecular geometry]] |
||
| 120°C–C–C<br>120° H–C–C <!-- Specify which angle, e.g. Cl-P-O. Please omit if not applicable --> |
| 120 °C–C–C<br>120° H–C–C <!-- Specify which angle, e.g. Cl-P-O. Please omit if not applicable --> |
||
|- |
|- |
||
| [[Magnetic susceptibility]] |
| [[Magnetic susceptibility]] |
||
| Line 35: | Line 36: | ||
|- |
|- |
||
| [[Surface tension]] |
| [[Surface tension]] |
||
| 28.88 dyn/cm at 25°C |
| 28.88 dyn/cm at 25 °C |
||
|- |
|- |
||
| [[Viscosity]]<ref name="cheric_p">{{Cite web|url= |
| [[Viscosity]]<ref name="cheric_p">{{Cite web|url=https://www.cheric.org/research/kdb/hcprop/showcoef.php?prop=VSL|title=Pure Component Properties|publisher=Chemical Engineering Research Information Center|accessdate=12 May 2007|format=Queriable database}}</ref> |
||
| |
| |
||
{| |
{| |
||
|- |
|- |
||
| 0.7528 mPa·s || at 10°C |
| 0.7528 mPa·s || at 10 °C |
||
|- |
|- |
||
| 0.6999 mPa·s || at 15°C |
| 0.6999 mPa·s || at 15 °C |
||
|- |
|- |
||
| 0.6516 mPa·s || at 20°C |
| 0.6516 mPa·s || at 20 °C |
||
|- |
|- |
||
| 0.6076 mPa·s || at 25°C |
| 0.6076 mPa·s || at 25 °C |
||
|- |
|- |
||
| 0.5673 mPa·s || at 35°C |
| 0.5673 mPa·s || at 35 °C |
||
|- |
|- |
||
| 0.4965 mPa·s || at 40°C |
| 0.4965 mPa·s || at 40 °C |
||
|- |
|- |
||
| 0.4655 mPa·s || at 45°C |
| 0.4655 mPa·s || at 45 °C |
||
|- |
|- |
||
| 0.4370 mPa·s || at 50°C |
| 0.4370 mPa·s || at 50 °C |
||
|- |
|- |
||
| 0.4108 mPa·s || at 55°C |
| 0.4108 mPa·s || at 55 °C |
||
|- |
|- |
||
| 0.3867 mPa·s || at 60°C |
| 0.3867 mPa·s || at 60 °C |
||
|- |
|- |
||
| 0.3644 mPa·s || at 65°C |
| 0.3644 mPa·s || at 65 °C |
||
|- |
|- |
||
| 0.3439 mPa·s || at 70°C |
| 0.3439 mPa·s || at 70 °C |
||
|- |
|- |
||
| 0.3250 mPa·s || at 75°C |
| 0.3250 mPa·s || at 75 °C |
||
|- |
|- |
||
| 0.3075 mPa·s || at 80°C |
| 0.3075 mPa·s || at 80 °C |
||
|- |
|- |
||
|} |
|} |
||
| Line 79: | Line 80: | ||
|- |
|- |
||
| [[Triple point]] |
| [[Triple point]] |
||
| 278.5 K (5.4 |
| 278.5 K (5.4 °C), 4.83 kPa |
||
|- |
|- |
||
| [[Critical point (chemistry)|Critical point]] |
| [[Critical point (chemistry)|Critical point]] |
||
| 562 K (289 |
| 562 K (289 °C), 4.89 MPa |
||
|- |
|- |
||
| [[Standard enthalpy change of fusion|Std enthalpy change<br/>of fusion]], Δ<sub>fus</sub>''H''<sup><s>o</s></sup> |
| [[Standard enthalpy change of fusion|Std enthalpy change<br/>of fusion]], Δ<sub>fus</sub>''H''<sup><s>o</s></sup> |
||
| 9.9 kJ/mol at 5.42 |
| 9.9 kJ/mol at 5.42 °C |
||
|- |
|- |
||
| [[Standard entropy change of fusion|Std entropy change<br/>of fusion]], Δ<sub>fus</sub>''S''<sup><s>o</s></sup> |
| [[Standard entropy change of fusion|Std entropy change<br/>of fusion]], Δ<sub>fus</sub>''S''<sup><s>o</s></sup> |
||
| 35.5 J/(mol·K) at 5.42 |
| 35.5 J/(mol·K) at 5.42 °C |
||
|- |
|- |
||
| [[Standard enthalpy change of vaporization|Std enthalpy change<br/>of vaporization]], Δ<sub>vap</sub>''H''<sup><s>o</s></sup> |
| [[Standard enthalpy change of vaporization|Std enthalpy change<br/>of vaporization]], Δ<sub>vap</sub>''H''<sup><s>o</s></sup> |
||
| 33.9 kJ/mol at 25°C<br>30.77 kJ/mol at 80.1°C |
| 33.9 kJ/mol at 25 °C<br>30.77 kJ/mol at 80.1 °C |
||
|- |
|- |
||
| [[Standard entropy change of vaporization|Std entropy change<br/>of vaporization]], Δ<sub>vap</sub>''S''<sup><s>o</s></sup> |
| [[Standard entropy change of vaporization|Std entropy change<br/>of vaporization]], Δ<sub>vap</sub>''S''<sup><s>o</s></sup> |
||
| 113.6 J/(mol·K) at 25°C<br>87.1 J/(mol·K) at 80.1°C |
| 113.6 J/(mol·K) at 25 °C<br>87.1 J/(mol·K) at 80.1 °C |
||
|- |
|- |
||
! {{chembox header}} | Solid properties |
! {{chembox header}} | Solid properties |
||
| Line 105: | Line 106: | ||
|- |
|- |
||
| [[Heat capacity]], ''c<sub>p</sub>'' |
| [[Heat capacity]], ''c<sub>p</sub>'' |
||
| 118.4 J/(mol K) at 0°C |
| 118.4 J/(mol K) at 0 °C |
||
|- |
|- |
||
! {{chembox header}} | Liquid properties |
! {{chembox header}} | Liquid properties |
||
| Line 126: | Line 127: | ||
| +82.93 kJ/mol |
| +82.93 kJ/mol |
||
|- |
|- |
||
| [[Standard molar entropy]],<ref name="ddbonline_etp">{{Cite web|url=http://ddbonline.ddbst.de/EE/31%20ETP%20%28Entropy%29.shtml|title=ETP Entropy of Benzene|format=Queriable database|publisher=Dortmund Data Bank|accessdate=7 |
| [[Standard molar entropy]],<ref name="ddbonline_etp">{{Cite web|url=http://ddbonline.ddbst.de/EE/31%20ETP%20%28Entropy%29.shtml|title=ETP Entropy of Benzene|format=Queriable database|publisher=Dortmund Data Bank|accessdate=7 October 2011}}</ref><br/>''S''<sup><s>o</s></sup><sub>gas</sub> |
||
| 269.01 J/(mol K) |
| 269.01 J/(mol K) |
||
|- |
|- |
||
| [[Heat capacity]],<ref name="cheric_p" |
| [[Heat capacity]],<ref name="cheric_p"/> ''c<sub>p</sub>'' |
||
| 82.44 J/(mol K) at 25°C |
| 82.44 J/(mol K) at 25 °C |
||
|- |
|- |
||
| [[van der Waals equation|van der Waals' constants]]<ref name="lange1522">''Lange's Handbook of Chemistry'' 10th ed, pp |
| [[van der Waals equation|van der Waals' constants]]<ref name="lange1522">''Lange's Handbook of Chemistry'' 10th ed, pp. 1522–1524</ref> |
||
| a = 1823.9 L<sup>2</sup> kPa/mol<sup>2</sup><br> b = 0.1154 liter per mole |
| a = 1823.9 L<sup>2</sup> kPa/mol<sup>2</sup><br> b = 0.1154 liter per mole |
||
|- |
|- |
||
| Line 328: | Line 329: | ||
! {{chembox header}} | [[Infrared|IR]] |
! {{chembox header}} | [[Infrared|IR]] |
||
|- |
|- |
||
| Major absorption bands<ref name="aist">{{Cite web|url=http://www.aist.go.jp/RIODB/SDBS/cgi-bin/cre_index.cgi|title=Spectral Database for Organic Compounds|publisher=Advanced Industrial Science and Technology|format=Queriable database|accessdate=10 June 2007}}</ref> |
| Major absorption bands<ref name="aist">{{Cite web |url=http://www.aist.go.jp/RIODB/SDBS/cgi-bin/cre_index.cgi |title=Spectral Database for Organic Compounds |publisher=Advanced Industrial Science and Technology |format=Queriable database |accessdate=10 June 2007 |url-status=dead |archiveurl=https://web.archive.org/web/20060505120103/http://www.aist.go.jp/RIODB/SDBS/cgi-bin/cre_index.cgi |archivedate=5 May 2006 }}</ref> |
||
| |
| |
||
{| |
{| |
||
| Line 403: | Line 404: | ||
|- |
|- |
||
| '''Principal hazards''' |
| '''Principal hazards''' |
||
| *** Benzene is a [[carcinogen]] |
| *** Benzene is a [[carcinogen]] (cancer-causing agent). |
||
|- |
|- |
||
| |
| |
||
| Line 451: | Line 452: | ||
<references/> |
<references/> |
||
<!-- [http://webbook.nist.gov/chemistry/ NIST Standard Reference Database] --> |
<!-- [http://webbook.nist.gov/chemistry/ NIST Standard Reference Database] --> |
||
{{Chemical data page general note}} |
|||
| ⚫ | |||
Except where noted otherwise, data relate to [[standard ambient temperature and pressure]]. |
|||
[[wikipedia:Chemical infobox|Disclaimer]] applies. |
|||
| ⚫ | |||
[[Category:Chemical data pages]] |
[[Category:Chemical data pages]] |
||
[[Category: |
[[Category:Benzene|Data page]] |
||
[[Category:Chemical data pages cleanup]] |
|||
Latest revision as of 14:14, 14 February 2024
This page provides supplementary chemical data on benzene.
Material Safety Data Sheet
[edit]The handling of this chemical may incur notable safety precautions. It is highly recommended to seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source such as SIRI, and follow its directions. MSDS for benzene is available at AMOCO.
Structure and properties
[edit]| Structure and properties | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refractive index, nD | 1.5011 at 20 °C | ||||||||||||||||||||||||||||
| Abbe number | ? | ||||||||||||||||||||||||||||
| Dielectric constant, εr | (2.274 – 0.0020ΔT) ε0 (ΔT = T – 25 °C) | ||||||||||||||||||||||||||||
| Bond energy | ? | ||||||||||||||||||||||||||||
| Bond length | 1.39 Å C-C[1] | ||||||||||||||||||||||||||||
| Molecular geometry | 120 °C–C–C 120° H–C–C | ||||||||||||||||||||||||||||
| Magnetic susceptibility | ? | ||||||||||||||||||||||||||||
| Surface tension | 28.88 dyn/cm at 25 °C | ||||||||||||||||||||||||||||
| Viscosity[2] |
| ||||||||||||||||||||||||||||
Thermodynamic properties
[edit]| Phase behavior | |
|---|---|
| Triple point | 278.5 K (5.4 °C), 4.83 kPa |
| Critical point | 562 K (289 °C), 4.89 MPa |
| Std enthalpy change of fusion, ΔfusH |
9.9 kJ/mol at 5.42 °C |
| Std entropy change of fusion, ΔfusS |
35.5 J/(mol·K) at 5.42 °C |
| Std enthalpy change of vaporization, ΔvapH |
33.9 kJ/mol at 25 °C 30.77 kJ/mol at 80.1 °C |
| Std entropy change of vaporization, ΔvapS |
113.6 J/(mol·K) at 25 °C 87.1 J/(mol·K) at 80.1 °C |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
45.56 J/(mol K) |
| Heat capacity, cp | 118.4 J/(mol K) at 0 °C |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
+48.7 kJ/mol |
| Standard molar entropy, S |
173.26 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–3273 kJ/mol |
| Heat capacity,[2] cp | 134.8 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
+82.93 kJ/mol |
| Standard molar entropy,[3] S |
269.01 J/(mol K) |
| Heat capacity,[2] cp | 82.44 J/(mol K) at 25 °C |
| van der Waals' constants[4] | a = 1823.9 L2 kPa/mol2 b = 0.1154 liter per mole |
Vapor pressure of liquid
[edit]| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15200 | 30400 | 45600 | |
| T in °C | –36.7(s) | –11.5(s) | 7.6 | 26.1 | 60.6 | 80.1 | 103.8 | 142.5 | 178.8 | 221.5 | 272.3 | — | |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed. Note: (s) notation indicates equilibrium temperature of vapor over solid, otherwise value is equilibrium temperature of vapor over liquid.

Distillation data
[edit]
|
|
|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Spectral data
[edit]| UV-Vis | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ionization potential | 9.24 eV (74525.6 cm−1) | ||||||||||||||||||||||||||
| S1 | 4.75 eV (38311.3 cm−1) | ||||||||||||||||||||||||||
| S2 | 6.05 eV (48796.5 cm−1) | ||||||||||||||||||||||||||
| λmax | 255 nm | ||||||||||||||||||||||||||
| Extinction coefficient, ε | ? | ||||||||||||||||||||||||||
| IR | |||||||||||||||||||||||||||
| Major absorption bands[6] |
| ||||||||||||||||||||||||||
| NMR | |||||||||||||||||||||||||||
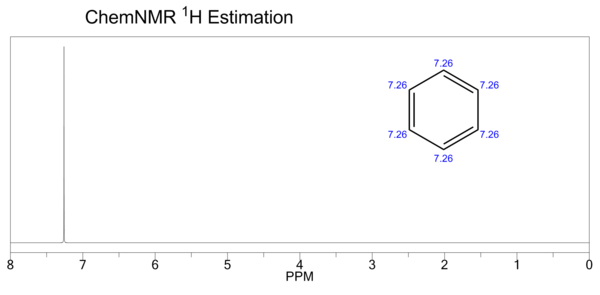
| Proton NMR | (CDCl3, 300 MHz) δ 7.34 (s, 6H) | ||||||||||||||||||||||||||
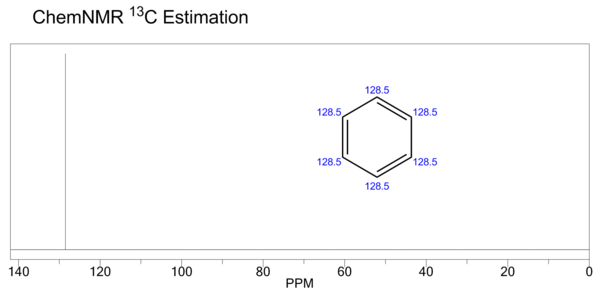
| Carbon-13 NMR | (CDCl3, 25 MHz) δ 128.4 | ||||||||||||||||||||||||||
| Other NMR data | |||||||||||||||||||||||||||
| MS | |||||||||||||||||||||||||||
| Masses of main fragments |
|||||||||||||||||||||||||||
Safety data
[edit]Material Safety Data Sheet for benzene:
| Common synonyms | None |
| Physical properties | Form: colorless liquid |
| Stability: Stable, but very flammable | |
| Melting point: 5.5 C | |
| Water solubility: negligible | |
| Specific gravity: 0.87 | |
| Principal hazards | *** Benzene is a carcinogen (cancer-causing agent). |
| *** Very flammable. The pure material, and any solutions containing it, constitute a fire risk. | |
| Safe handling | Benzene should NOT be used at all unless no safer alternatives are available. |
| If benzene must be used in an experiment, it should be handled at all stages in a fume cupboard. | |
| Wear safety glasses and use protective gloves. | |
| Emergency | Eye contact: Immediately flush the eye with plenty of water. Continue for at least ten minutes |
| and call for immediate medical help. | |
| Skin contact: Wash off with soap and water. Remove any contaminated clothing. If the skin | |
| reddens or appears damaged, call for medical aid. | |
| If swallowed: Call for immediate medical help. | |
| Disposal | It is dangerous to try to dispose of benzene by washing it down a sink, since it is toxic, will cause environmental damage |
| and presents a fire risk. It is probable that trying to dispose of benzene in this way will also break local | |
| environmental rules. Instead, retain in a safe place in the laboratory (well away from any source of ignition) | |
| for disposal with other flammable, non-chlorinated solvents. | |
| Protective equipment | Safety glasses. If gloves are worn, PVA, butyl rubber and viton are suitable materials. |
References
[edit]- ^ Brown; LeMay; Bursten (2006). Chemistry: The Central Science. Upper Saddle River, NJ: Pearson Education. pp. 1067. ISBN 0-13-109686-9.
- ^ a b c d "Pure Component Properties" (Queriable database). Chemical Engineering Research Information Center. Retrieved 12 May 2007.
- ^ "ETP Entropy of Benzene" (Queriable database). Dortmund Data Bank. Retrieved 7 October 2011.
- ^ Lange's Handbook of Chemistry 10th ed, pp. 1522–1524
- ^ a b c d "Binary Vapor-Liquid Equilibrium Data" (Queriable database). Chemical Engineering Research Information Center. Retrieved 12 May 2007.
- ^ "Spectral Database for Organic Compounds". Advanced Industrial Science and Technology. Archived from the original (Queriable database) on 5 May 2006. Retrieved 10 June 2007.
This box:
- Except where noted otherwise, data relate to Standard temperature and pressure.
- Reliability of data general note.