Tin(IV) iodide: Difference between revisions
No edit summary |
Altered template type. Add: bibcode, series, authors 1-1. Removed parameters. Some additions/deletions were parameter name changes. | Use this tool. Report bugs. | #UCB_Gadget |
||
| (8 intermediate revisions by 7 users not shown) | |||
| Line 45: | Line 45: | ||
| CrystalStruct = [[Cubic crystal system|Cubic]], [[Pearson symbol|''cP40'']] |
| CrystalStruct = [[Cubic crystal system|Cubic]], [[Pearson symbol|''cP40'']] |
||
| SpaceGroup = Pa-3 No. 205 }} |
| SpaceGroup = Pa-3 No. 205 }} |
||
| Section9 = {{Chembox Related |
|||
| OtherAnions = [[Tin(IV) fluoride]] <br> [[Tin(IV) chloride]] <br> [[Tin(IV) bromide]] |
|||
| OtherCations = [[Carbon tetraiodide]] <br> [[Silicon tetraiodide]] <br> [[Germanium tetraiodide]] |
|||
| OtherFunction = |
|||
| OtherFunction_label = |
|||
| OtherCompounds = |
|||
}} |
|||
}} |
}} |
||
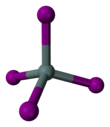
'''Tin(IV) iodide''', also known as '''stannic iodide''', is the [[chemical compound]] with the [[chemical formula|formula]] SnI<sub>4</sub>. This tetrahedral molecule crystallizes as a bright orange solid that dissolves readily in nonpolar solvents such as [[benzene]].<ref>[http://www.webelements.com/webelements/compounds/text/Sn/I4Sn1-7790478.html Chemistry : Periodic Table : tin : compound data [tin (IV) iodide]<!-- Bot generated title -->]</ref> |
'''Tin(IV) iodide''', also known as '''stannic iodide''', is the [[chemical compound]] with the [[chemical formula|formula]] SnI<sub>4</sub>. This tetrahedral molecule crystallizes as a bright orange solid that dissolves readily in nonpolar solvents such as [[benzene]].<ref>[http://www.webelements.com/webelements/compounds/text/Sn/I4Sn1-7790478.html Chemistry : Periodic Table : tin : compound data [tin (IV) iodide]<!-- Bot generated title -->]</ref> |
||
== Preparation == |
|||
The compound is usually prepared by the reaction of [[iodine]] and [[tin]]:<ref name=Moeller>{{cite journal |last1= Moeller|first1=T.|last2=Edwards|first2=D. C.|title= Tin(IV) Iodide (Stannic Iodide) |journal= [[Inorganic Syntheses]] |year= 1953 |volume= 4 |pages= 119–121 |doi= 10.1002/9780470132357.ch40}}</ref> |
|||
:[[tin|Sn]] + 2 [[iodine|I<sub>2</sub>]] → SnI<sub>4</sub> |
|||
The compound [[ |
The compound is usually prepared by the reaction of [[iodine]] and [[tin]]:<ref name=Moeller>{{cite book |last1= Moeller|first1=T.|last2=Edwards|first2=D. C.|title= Tin(IV) Iodide (Stannic Iodide) |series= [[Inorganic Syntheses]] |year= 1953 |volume= 4 |pages= 119–121 |doi= 10.1002/9780470132357.ch40}}</ref> |
||
:{{chem2|Sn + 2I2 -> SnI4}} |
|||
== Chemical properties == |
|||
The compound [[hydrolysis|hydrolyses]] in water.<ref name=Hickling /> In aqueous [[hydroiodic acid]], it reacts to form a rare example of a hexaiodometallate:<ref name=Moeller/> |
|||
: SnI<sub>4</sub> + 2 [[iodide|I<sup>−</sup>]] → [SnI<sub>6</sub>]<sup>2−</sup> |
: SnI<sub>4</sub> + 2 [[iodide|I<sup>−</sup>]] → [SnI<sub>6</sub>]<sup>2−</sup> |
||
== Physical properties == |
|||
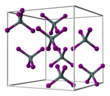
Tin(IV) iodide is an orange solid under standard conditions.<ref name=Hickling>{{Cite journal |last=Hickling |first=George G. |date=Aug 1990 |title=Gravimetric analysis: The synthesis of tin iodide |url=https://pubs.acs.org/doi/abs/10.1021/ed067p702 |journal=Journal of Chemical Education |language=en |volume=67 |issue=8 |pages=702 |doi=10.1021/ed067p702 |bibcode=1990JChEd..67..702H |issn=0021-9584}}</ref> It has a cubic crystal structure with the [[space group]] ''Pa''{{overline|3}} (space group no. 205), the lattice parameter a = 1226 pm and eight formula units per [[unit cell]].<ref>{{Cite journal |last1=Meller |first1=F. |last2=Fankuchen |first2=I. |date=1955-06-10 |title=The crystal structure of tin tetraiodide |url=https://scripts.iucr.org/cgi-bin/paper?S0365110X55001035 |journal=Acta Crystallographica |language=en |volume=8 |issue=6 |pages=343–344 |doi=10.1107/S0365110X55001035 |issn=0365-110X|doi-access=free |bibcode=1955AcCry...8..343M }}</ref> This corresponds approximately to a cubic close packing of iodine atoms in which 1/8 of all tetrahedral gaps are occupied by tin atoms. This leads to discrete tetrahedral SnI<sub>4</sub> molecules.<ref>{{Cite book |last1=Wiberg |first1=Egon |title=Lehrbuch der anorganischen Chemie |last2=Wiberg |first2=Nils |date=2007 |publisher=Walter de Gruyter |isbn=978-3-11-017770-1 |editor-last=Holleman |editor-first=Arnold F. |edition=102., stark umgearbeitete und verbesserte Auflage |location=Berlin New York |editor-last2=Fischer |editor-first2=Gerd}}</ref> |
|||
==See also== |
==See also== |
||
| Line 62: | Line 79: | ||
{{Tin compounds}} |
{{Tin compounds}} |
||
{{Iodides}} |
|||
[[Category:Tin compounds]] |
[[Category:Tin(IV) compounds]] |
||
[[Category:Iodides]] |
[[Category:Iodides]] |
||
[[Category:Metal halides]] |
[[Category:Metal halides]] |
||
Latest revision as of 22:59, 17 February 2024
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
tin(IV) iodide
| |||
| Other names
tin tetraiodide
stannic iodide | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.029.281 | ||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| SnI4 | |||
| Molar mass | 626.328 g mol−1 | ||
| Appearance | red-orange solid | ||
| Density | 4.56 g cm−3 | ||
| Melting point | 143 °C (289 °F; 416 K) | ||
| Boiling point | 348.5 °C (659.3 °F; 621.6 K) | ||
Refractive index (nD)
|
2.106 | ||
| Structure | |||
| Cubic, cP40 | |||
| Pa-3 No. 205 | |||
| Related compounds | |||
Other anions
|
Tin(IV) fluoride Tin(IV) chloride Tin(IV) bromide | ||
Other cations
|
Carbon tetraiodide Silicon tetraiodide Germanium tetraiodide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Tin(IV) iodide, also known as stannic iodide, is the chemical compound with the formula SnI4. This tetrahedral molecule crystallizes as a bright orange solid that dissolves readily in nonpolar solvents such as benzene.[1]
Preparation
[edit]The compound is usually prepared by the reaction of iodine and tin:[2]
- Sn + 2I2 → SnI4
Chemical properties
[edit]The compound hydrolyses in water.[3] In aqueous hydroiodic acid, it reacts to form a rare example of a hexaiodometallate:[2]
- SnI4 + 2 I− → [SnI6]2−
Physical properties
[edit]Tin(IV) iodide is an orange solid under standard conditions.[3] It has a cubic crystal structure with the space group Pa3 (space group no. 205), the lattice parameter a = 1226 pm and eight formula units per unit cell.[4] This corresponds approximately to a cubic close packing of iodine atoms in which 1/8 of all tetrahedral gaps are occupied by tin atoms. This leads to discrete tetrahedral SnI4 molecules.[5]
See also
[edit]References
[edit]- ^ Chemistry : Periodic Table : tin : compound data [tin (IV) iodide]
- ^ a b Moeller, T.; Edwards, D. C. (1953). Tin(IV) Iodide (Stannic Iodide). Inorganic Syntheses. Vol. 4. pp. 119–121. doi:10.1002/9780470132357.ch40.
- ^ a b Hickling, George G. (Aug 1990). "Gravimetric analysis: The synthesis of tin iodide". Journal of Chemical Education. 67 (8): 702. Bibcode:1990JChEd..67..702H. doi:10.1021/ed067p702. ISSN 0021-9584.
- ^ Meller, F.; Fankuchen, I. (1955-06-10). "The crystal structure of tin tetraiodide". Acta Crystallographica. 8 (6): 343–344. Bibcode:1955AcCry...8..343M. doi:10.1107/S0365110X55001035. ISSN 0365-110X.
- ^ Wiberg, Egon; Wiberg, Nils (2007). Holleman, Arnold F.; Fischer, Gerd (eds.). Lehrbuch der anorganischen Chemie (102., stark umgearbeitete und verbesserte Auflage ed.). Berlin New York: Walter de Gruyter. ISBN 978-3-11-017770-1.