Montréalone: Difference between revisions
Fixed reference date error(s) (see CS1 errors: dates for details) and AWB general fixes |
|||
| (17 intermediate revisions by 11 users not shown) | |||
| Line 1: | Line 1: | ||
{{Chembox |
|||
'''Montréalone''' (synonyms: montrealone, phospha-münchnone) is a [[mesoionic]] [[heterocyclic compound|heterocyclic]] [[chemical compound]]. |
|||
| ImageFile = Montrealone parent structure.png |
|||
| ImageSize = 100px |
|||
| ImageCaption = Montréalone parent compound |
|||
| IUPACName = |
|||
<!-- more general Chembox parameters here --> |
|||
| Section1 = {{Chembox Identifiers |
|||
| CASNo = 1228992-40-2 |
|||
| CASNo_Ref = {{cascite|correct|CAS}} |
|||
| DTXSID = DTXSID701018796 |
|||
| PubChem = 154735194 |
|||
| StdInChI=1S/C2H5NOP/c1-3-2-5-4-1/h1-3H,5H2/q+1 |
|||
| StdInChIKey = CEHNCPLHGNXBLW-UHFFFAOYSA-N |
|||
| SMILES = [CH-]1[NH+]=CO[PH2+]1 |
|||
}} |
|||
}} |
|||
'''Montréalone''' (synonyms: montrealone, phospha-münchnone) is a [[mesoionic]] [[heterocyclic compound|heterocyclic]] [[chemical compound]]. It is named for the city of [[Montreal|Montréal]], Canada, which is the location of [[McGill University]], where it was first discovered.<ref>{{Cite journal |last1=Reissig |first1=Hans-Ulrich |last2=Zimmer |first2=Reinhold |date=2014-09-08 |title=Münchnones-New Facets after 50 Years |url=https://onlinelibrary.wiley.com/doi/10.1002/anie.201405092 |journal=Angewandte Chemie International Edition |language=en |volume=53 |issue=37 |pages=9708–9710 |doi=10.1002/anie.201405092|pmid=25045012 }}</ref> |
|||
== Structure == |
== Structure == |
||
The montréalone [[parent structure|parent compound]] has been |
The montréalone [[parent structure|parent compound]] has been studied theoretically,<ref name="krenske2008">{{Cite journal |last=Krenske |first=Elizabeth H. |last2=Houk |first2=K. N. |last3=Arndtsen |first3=Bruce A. |last4=St. Cyr |first4=Daniel J. |date=2008-08-01 |title=Cyclic 1,3-Dipoles or Acyclic Phosphonium Ylides? Electronic Characterization of "Montréalones" |url=https://pubs.acs.org/doi/10.1021/ja802646f |journal=Journal of the American Chemical Society |language=en |volume=130 |issue=31 |pages=10052–10053 |doi=10.1021/ja802646f |issn=0002-7863}}</ref> and is unlikely to exist as a stable species. [[structural analog|Analogs]] bearing multiple [[substituent]]s display [[chemical stability|stability]] suitable for synthesis, isolation, and characterization.<ref name="St-Cyr2010">{{Cite journal |last=St-Cyr |first=Daniel J. |last2=Morin |first2=Marie S. T. |last3=Bélanger-Gariépy |first3=Francine |last4=Arndtsen |first4=Bruce A. |last5=Krenske |first5=Elizabeth H. |last6=Houk |first6=K. N. |date=2010-06-18 |title=Phospha-Münchnones: Electronic Structures and 1,3-Dipolar Cycloadditions |url=https://pubs.acs.org/doi/10.1021/jo1008383 |journal=The Journal of Organic Chemistry |language=en |volume=75 |issue=12 |pages=4261–4273 |doi=10.1021/jo1008383 |issn=0022-3263}}</ref> Substituted montréalones with a balance of [[chemical stability|stability]] and [[Reactivity (chemistry)|reactivity]] have been used as [[reaction intermediate]]s in the synthesis of other [[heterocyclic compound|heterocycles]]. |
||
[[File:substituted montrealone.png|139x104 px|center|A substituted montréalone]] |
[[File:substituted montrealone.png|139x104 px|center|A substituted montréalone]] |
||
Theoretical and experimental analysis of [[chemical stability|stable]] montréalones reveals overlapping [[azomethine ylide]] and [[Wittig reaction#Wittig reagents|Wittig-type]] moieties within the [[Saturated and unsaturated compounds|unsaturated]] 5-membered [[wikt: organophosphorus|organophosphorus]] ring system. Depending on the phosphorus substituents, [[Tautomer#Valence tautomerism|ring-chain valence tautomerism]] allows montréalones to display variable degrees of equilibrium and structural blending with [[N-Acylamides|''N''-acyl amino]] [[Ylide#Phosphonium ylides|phosphonium ylide]] forms. The cyclic [[1,3-dipole|1,3-dipolar]] form is detectably or exclusively formed with [[Phosphite#Synthesis of phosphite esters|phosphite]] and [[phosphonite]]-based [[structural analog|analogs]]. [[Triphenylphosphine]]-based variants lack sufficient tendency for intramolecular cyclization, and exist as acyclic [[Ylide#Phosphonium ylides|phosphonium ylides]].<ref name="krenske2008" /><ref name="St-Cyr2010" /><ref name="St-Cyr2007">{{Cite journal |last=St. Cyr |first=Daniel J. |last2=Arndtsen |first2=Bruce A. |date=2007-10-01 |title=A New Use of Wittig-Type Reagents as 1,3-Dipolar Cycloaddition Precursors and in Pyrrole Synthesis |url=https://pubs.acs.org/doi/10.1021/ja074330w |journal=Journal of the American Chemical Society |language=en |volume=129 |issue=41 |pages=12366–12367 |doi=10.1021/ja074330w |issn=0002-7863}}</ref><ref>{{Cite journal |last=Reissig |first=Hans‐Ulrich |last2=Zimmer |first2=Reinhold |date=2014-09-08 |title=Münchnones—New Facets after 50 Years |url=https://onlinelibrary.wiley.com/doi/10.1002/anie.201405092 |journal=Angewandte Chemie International Edition |language=en |volume=53 |issue=37 |pages=9708–9710 |doi=10.1002/anie.201405092 |issn=1433-7851}}</ref> |
|||
[[File:Montrealone resonance and equilibrium structures.png|797x73 px|center]] |
[[File:Montrealone resonance and equilibrium structures.png|797x73 px|center]] |
||
| Line 12: | Line 27: | ||
== Synthesis == |
== Synthesis == |
||
Montréalones |
Montréalones are related to [[Wittig reaction#Wittig reagents|Wittig reagents]] and can be generated in a similar fashion. Reaction of [[Organophosphorus compound#Organophosphorus.28III.29 compounds.2C main categories|organophosphorus(III) compounds]] with ''N''-acyliminium ions affords [[phosphonium salt]] intermediates which are [[deprotonation|deprotonated]] using non-nucleophilic bases (e.g. [[1,8-Diazabicycloundec-7-ene|DBU]], [[Lithium bis(trimethylsilyl)amide|LiHMDS]]). ''N''-Acyliminium ions are generated [[In situ#Chemistry and chemical engineering|in situ]] from [[acyl chloride|acid chlorides]] and [[imine]]s such that the '[[1,3-dipole]]' itself is generated in a [[multi-component reaction]]. The most efficient [[Organophosphorus compound#Organophosphorus.28III.29 compounds.2C main categories|phosphorus(III)]] precursor for [[1,3-dipole|dipole]] synthesis is 2-phenylbenzo[d][1,3,2]dioxaphosphole [PhP([[catechol|catechyl]])] owing to the balance of [[nucleophile|nucleophilicity]] and [[electrophile|electrophilicity]] that it affords.<ref name="St-Cyr2007" /> |
||
[[File:Montrealone generation.png|797x73 px|center]] |
[[File:Montrealone generation.png|797x73 px|center]] |
||
| Line 18: | Line 33: | ||
== Reactions == |
== Reactions == |
||
Montréalones |
Montréalones participate in [[1,3-dipolar cycloaddition]] reactions with [[wikt:dipolarophile|dipolarophiles]] such as [[imine]]s, [[alkene]]s, and [[alkyne]]s to respectively afford [[imidazole]]s,<ref>{{Cite journal |last=Aly |first=Sara |last2=Romashko |first2=Mikhail |last3=Arndtsen |first3=Bruce A. |date=2015-03-06 |title=Multicomponent Synthesis of Substituted and Fused-Ring Imidazoles via Phospha-münchnone Cycloaddition |url=https://pubs.acs.org/doi/10.1021/jo5028936 |journal=The Journal of Organic Chemistry |language=en |volume=80 |issue=5 |pages=2709–2714 |doi=10.1021/jo5028936 |issn=0022-3263}}</ref> 2-[[pyrroline]]s,<ref>{{Cite journal |last=Morin |first=Marie S. T. |last2=Arndtsen |first2=Bruce A. |date=2014-02-21 |title=Chiral Phosphorus-Based 1,3-Dipoles: A Modular Approach to Enantioselective 1,3-Dipolar Cycloaddition and Polycyclic 2-Pyrroline Synthesis |url=https://pubs.acs.org/doi/10.1021/ol4035512 |journal=Organic Letters |language=en |volume=16 |issue=4 |pages=1056–1059 |doi=10.1021/ol4035512 |issn=1523-7060}}</ref> and [[pyrrole]]s.<ref name="St-Cyr2010" /><ref>Organic Chemistry Portal pyrrole synthesis abstract: {{cite web |url=https://www.organic-chemistry.org/abstracts/lit1/886.shtm |title= A New Use of Wittig-Type Reagents as 1,3-Dipolar Cycloaddition Precursors and in Pyrrole Synthesis. |access-date=6 April 2015}}</ref> The latter [[heterocyclic compound|heterocyclic]] products were obtained by [[multi-component reaction]]s involving [[in situ]]-generation of montréalones from [[imine]]s, [[acyl chloride|acid chlorides]], and [[phosphonite]]s prior to [[One-pot synthesis|one-pot]] reactions with the [[wikt:dipolarophile|dipolarophile]]. Moreover, conjugated poly(1,3-dipole) variants of montréalones have been generated and used in the synthesis of [[conductive polymer|polyheterocycles]] related to [[polypyrrole]]s.<ref>{{Cite journal |last=Kayser |first=Laure V. |last2=Vollmer |first2=Moritz |last3=Welnhofer |first3=Merve |last4=Krikcziokat |first4=Hanna |last5=Meerholz |first5=Klaus |last6=Arndtsen |first6=Bruce A. |date=2016-08-24 |title=Metal-Free, Multicomponent Synthesis of Pyrrole-Based π-Conjugated Polymers from Imines, Acid Chlorides, and Alkynes |url=https://pubs.acs.org/doi/10.1021/jacs.6b05035 |journal=Journal of the American Chemical Society |language=en |volume=138 |issue=33 |pages=10516–10521 |doi=10.1021/jacs.6b05035 |issn=0002-7863}}</ref><ref>{{Cite journal |last=Kayser |first=Laure V. |last2=Hartigan |first2=Elizabeth M. |last3=Arndtsen |first3=Bruce A. |date=2016-12-05 |title=Multicomponent Coupling Approach to Cross-Conjugated Polymers from Vanillin-Based Monomers |url=https://pubs.acs.org/doi/10.1021/acssuschemeng.6b02302 |journal=ACS Sustainable Chemistry & Engineering |language=en |volume=4 |issue=12 |pages=6263–6267 |doi=10.1021/acssuschemeng.6b02302 |issn=2168-0485}}</ref> |
||
[[File:Montrealone reactions.png|320x179 px|center]] |
[[File:Montrealone reactions.png|320x179 px|center]] |
||
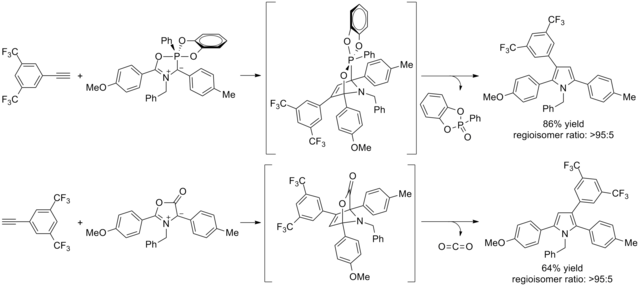
Cycloaddition reactions of asymmetric [[1,3-dipole]]s and dipolarophiles can lead to [[isomer]]ic product mixtures, particularly with [[münchnone]]s and [[alkyne]]s in the synthesis of [[pyrrole]]s.<ref>Lubell, W.; St-Cyr, D.; Dufour-Gallant, J.; Hopewell, R.; Boutard, N.; Kassem, T.; Dörr, A.; Zelli, R., {{cite web |url=https://www.thieme.de/en/thieme-chemistry/sos-knowledge-updates-2013-58727.htm |title= 1H-Pyrroles (Update 2013)}} ''Science of Synthesis'' '''2013''', ''2013/1'', 157-388.</ref><ref>Gribble |
Cycloaddition reactions of asymmetric [[1,3-dipole]]s and [[wikt:dipolarophile|dipolarophiles]] can lead to [[isomer]]ic product mixtures, particularly with [[münchnone]]s and [[alkyne]]s in the synthesis of [[pyrrole]]s.<ref>Lubell, W.; St-Cyr, D.; Dufour-Gallant, J.; Hopewell, R.; Boutard, N.; Kassem, T.; Dörr, A.; Zelli, R., {{cite web |url=https://www.thieme.de/en/thieme-chemistry/sos-knowledge-updates-2013-58727.htm |title= 1H-Pyrroles (Update 2013)}} ''Science of Synthesis'' '''2013''', ''2013/1'', 157-388.</ref><ref>{{Cite book |last=Gribble |first=Gordon W. |url=https://onlinelibrary.wiley.com/doi/10.1002/0471428035.ch4 |title=Mesoionic Oxazoles |date=2003-07-25 |publisher=John Wiley & Sons, Inc. |isbn=978-0-471-39494-5 |editor-last=Palmer |editor-first=David C. |volume=60 |location=Hoboken, NJ, USA |pages=473–576 |language=en |doi=10.1002/0471428035.ch4}}</ref><ref>{{Cite book |last=Gingrich |first=Henry L. |url=https://onlinelibrary.wiley.com/doi/10.1002/9780470187289.ch4 |title=Mesoionic Oxazoles |last2=Baum |first2=Jonathan S. |date=January 1986 |publisher=Wiley |isbn=978-0-471-86958-0 |editor-last=Turchi |editor-first=I. J. |edition=1 |volume=45 |pages=731–961 |language=en |doi=10.1002/9780470187289.ch4}}</ref> In contrast to related [[Diels-Alder reactions]], rationalization of [[wikt:regioisomeric|regioisomeric]] bias using conventional [[Diels–Alder reaction#Regioselectivity|frontier molecular orbital (FMO) theory]] fails. The complementary use of montéalones and münchnones allows product mixtures to be avoided and highlights the need to consider [[Transition state|transition-state]] geometrical changes (distortion) in the rationalization process.<ref>{{Cite journal |last=Morin |first=Marie S. T. |last2=St-Cyr |first2=Daniel J. |last3=Arndtsen |first3=Bruce A. |last4=Krenske |first4=Elizabeth H. |last5=Houk |first5=K. N. |date=2013-11-20 |title=Modular Mesoionics: Understanding and Controlling Regioselectivity in 1,3-Dipolar Cycloadditions of Münchnone Derivatives |url=https://pubs.acs.org/doi/10.1021/ja406833q |journal=Journal of the American Chemical Society |language=en |volume=135 |issue=46 |pages=17349–17358 |doi=10.1021/ja406833q |issn=0002-7863}}</ref> |
||
[[File:Montrealone vs munchnone cycloaddition.png|640x287 px|center]] |
[[File:Montrealone vs munchnone cycloaddition.png|640x287 px|center]] |
||
| Line 36: | Line 51: | ||
{{DEFAULTSORT:Montrealone}} |
{{DEFAULTSORT:Montrealone}} |
||
[[Category: |
[[Category:Oxygen heterocycles]] |
||
[[Category:Phosphorus heterocycles]] |
|||
[[Category:Nitrogen heterocycles]] |
|||
[[Category:Pentacyclic compounds]] |
|||
[[Category:Phosphine oxides]] |
|||
Latest revision as of 19:37, 21 February 2024
 Montréalone parent compound
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Montréalone (synonyms: montrealone, phospha-münchnone) is a mesoionic heterocyclic chemical compound. It is named for the city of Montréal, Canada, which is the location of McGill University, where it was first discovered.[1]
Structure
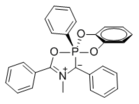
[edit]The montréalone parent compound has been studied theoretically,[2] and is unlikely to exist as a stable species. Analogs bearing multiple substituents display stability suitable for synthesis, isolation, and characterization.[3] Substituted montréalones with a balance of stability and reactivity have been used as reaction intermediates in the synthesis of other heterocycles.
Theoretical and experimental analysis of stable montréalones reveals overlapping azomethine ylide and Wittig-type moieties within the unsaturated 5-membered organophosphorus ring system. Depending on the phosphorus substituents, ring-chain valence tautomerism allows montréalones to display variable degrees of equilibrium and structural blending with N-acyl amino phosphonium ylide forms. The cyclic 1,3-dipolar form is detectably or exclusively formed with phosphite and phosphonite-based analogs. Triphenylphosphine-based variants lack sufficient tendency for intramolecular cyclization, and exist as acyclic phosphonium ylides.[2][3][4][5]

Synthesis
[edit]Montréalones are related to Wittig reagents and can be generated in a similar fashion. Reaction of organophosphorus(III) compounds with N-acyliminium ions affords phosphonium salt intermediates which are deprotonated using non-nucleophilic bases (e.g. DBU, LiHMDS). N-Acyliminium ions are generated in situ from acid chlorides and imines such that the '1,3-dipole' itself is generated in a multi-component reaction. The most efficient phosphorus(III) precursor for dipole synthesis is 2-phenylbenzo[d][1,3,2]dioxaphosphole [PhP(catechyl)] owing to the balance of nucleophilicity and electrophilicity that it affords.[4]

Reactions
[edit]Montréalones participate in 1,3-dipolar cycloaddition reactions with dipolarophiles such as imines, alkenes, and alkynes to respectively afford imidazoles,[6] 2-pyrrolines,[7] and pyrroles.[3][8] The latter heterocyclic products were obtained by multi-component reactions involving in situ-generation of montréalones from imines, acid chlorides, and phosphonites prior to one-pot reactions with the dipolarophile. Moreover, conjugated poly(1,3-dipole) variants of montréalones have been generated and used in the synthesis of polyheterocycles related to polypyrroles.[9][10]

Cycloaddition reactions of asymmetric 1,3-dipoles and dipolarophiles can lead to isomeric product mixtures, particularly with münchnones and alkynes in the synthesis of pyrroles.[11][12][13] In contrast to related Diels-Alder reactions, rationalization of regioisomeric bias using conventional frontier molecular orbital (FMO) theory fails. The complementary use of montéalones and münchnones allows product mixtures to be avoided and highlights the need to consider transition-state geometrical changes (distortion) in the rationalization process.[14]
See also
[edit]References
[edit]- ^ Reissig, Hans-Ulrich; Zimmer, Reinhold (2014-09-08). "Münchnones-New Facets after 50 Years". Angewandte Chemie International Edition. 53 (37): 9708–9710. doi:10.1002/anie.201405092. PMID 25045012.
- ^ a b Krenske, Elizabeth H.; Houk, K. N.; Arndtsen, Bruce A.; St. Cyr, Daniel J. (2008-08-01). "Cyclic 1,3-Dipoles or Acyclic Phosphonium Ylides? Electronic Characterization of "Montréalones"". Journal of the American Chemical Society. 130 (31): 10052–10053. doi:10.1021/ja802646f. ISSN 0002-7863.
- ^ a b c St-Cyr, Daniel J.; Morin, Marie S. T.; Bélanger-Gariépy, Francine; Arndtsen, Bruce A.; Krenske, Elizabeth H.; Houk, K. N. (2010-06-18). "Phospha-Münchnones: Electronic Structures and 1,3-Dipolar Cycloadditions". The Journal of Organic Chemistry. 75 (12): 4261–4273. doi:10.1021/jo1008383. ISSN 0022-3263.
- ^ a b St. Cyr, Daniel J.; Arndtsen, Bruce A. (2007-10-01). "A New Use of Wittig-Type Reagents as 1,3-Dipolar Cycloaddition Precursors and in Pyrrole Synthesis". Journal of the American Chemical Society. 129 (41): 12366–12367. doi:10.1021/ja074330w. ISSN 0002-7863.
- ^ Reissig, Hans‐Ulrich; Zimmer, Reinhold (2014-09-08). "Münchnones—New Facets after 50 Years". Angewandte Chemie International Edition. 53 (37): 9708–9710. doi:10.1002/anie.201405092. ISSN 1433-7851.
- ^ Aly, Sara; Romashko, Mikhail; Arndtsen, Bruce A. (2015-03-06). "Multicomponent Synthesis of Substituted and Fused-Ring Imidazoles via Phospha-münchnone Cycloaddition". The Journal of Organic Chemistry. 80 (5): 2709–2714. doi:10.1021/jo5028936. ISSN 0022-3263.
- ^ Morin, Marie S. T.; Arndtsen, Bruce A. (2014-02-21). "Chiral Phosphorus-Based 1,3-Dipoles: A Modular Approach to Enantioselective 1,3-Dipolar Cycloaddition and Polycyclic 2-Pyrroline Synthesis". Organic Letters. 16 (4): 1056–1059. doi:10.1021/ol4035512. ISSN 1523-7060.
- ^ Organic Chemistry Portal pyrrole synthesis abstract: "A New Use of Wittig-Type Reagents as 1,3-Dipolar Cycloaddition Precursors and in Pyrrole Synthesis". Retrieved 6 April 2015.
- ^ Kayser, Laure V.; Vollmer, Moritz; Welnhofer, Merve; Krikcziokat, Hanna; Meerholz, Klaus; Arndtsen, Bruce A. (2016-08-24). "Metal-Free, Multicomponent Synthesis of Pyrrole-Based π-Conjugated Polymers from Imines, Acid Chlorides, and Alkynes". Journal of the American Chemical Society. 138 (33): 10516–10521. doi:10.1021/jacs.6b05035. ISSN 0002-7863.
- ^ Kayser, Laure V.; Hartigan, Elizabeth M.; Arndtsen, Bruce A. (2016-12-05). "Multicomponent Coupling Approach to Cross-Conjugated Polymers from Vanillin-Based Monomers". ACS Sustainable Chemistry & Engineering. 4 (12): 6263–6267. doi:10.1021/acssuschemeng.6b02302. ISSN 2168-0485.
- ^ Lubell, W.; St-Cyr, D.; Dufour-Gallant, J.; Hopewell, R.; Boutard, N.; Kassem, T.; Dörr, A.; Zelli, R., "1H-Pyrroles (Update 2013)". Science of Synthesis 2013, 2013/1, 157-388.
- ^ Gribble, Gordon W. (2003-07-25). Palmer, David C. (ed.). Mesoionic Oxazoles. Vol. 60. Hoboken, NJ, USA: John Wiley & Sons, Inc. pp. 473–576. doi:10.1002/0471428035.ch4. ISBN 978-0-471-39494-5.
- ^ Gingrich, Henry L.; Baum, Jonathan S. (January 1986). Turchi, I. J. (ed.). Mesoionic Oxazoles. Vol. 45 (1 ed.). Wiley. pp. 731–961. doi:10.1002/9780470187289.ch4. ISBN 978-0-471-86958-0.
- ^ Morin, Marie S. T.; St-Cyr, Daniel J.; Arndtsen, Bruce A.; Krenske, Elizabeth H.; Houk, K. N. (2013-11-20). "Modular Mesoionics: Understanding and Controlling Regioselectivity in 1,3-Dipolar Cycloadditions of Münchnone Derivatives". Journal of the American Chemical Society. 135 (46): 17349–17358. doi:10.1021/ja406833q. ISSN 0002-7863.
