Allylic rearrangement: Difference between revisions
m AWB assisted change "a" to "an". |
Rewrite and rearrange for concicions and general organization. Also add cite to Patai |
||
| (44 intermediate revisions by 35 users not shown) | |||
| Line 1: | Line 1: | ||
An '''allylic rearrangement''' or '''allylic shift''' is an [[organic reaction|organic chemical reaction]] in which reaction at a center [[Vicinal (chemistry)|vicinal]] to a [[double bond]] causes the double bond to shift to an adjacent pair of atoms: |
|||
[[File:SN2_accent_reaction_mechanism.png|alt=SN2 accent reaction mechanism|center|400x400px]] |
|||
It is encountered in both [[nucleophilic substitution|nucleophilic]] and [[electrophilic substitution]], although it is usually [[Side reaction|suppressed]] relative to non-allylic substitution. For example, reaction of 1-chloro-2-butene with [[sodium hydroxide]] gives 2-buten-1-ol and 3-buten-2-ol: |
|||
[[File:AllylicRearrangementReaction.png|alt=reaction of 1-chloro-but-2-ene with sodium hydroxide|center|400x400px]] |
|||
| ⚫ | |||
Allylic shifts occur because the [[transition state]] is an [[Allyl group|allyl]] intermediate. In other respects they are similar to classical nucleophilic substitution, and admit both [[SN2 reaction|bimolecular]] and [[SN1 reaction|monomolecular]] mechanisms (respectively the '''S<sub>N</sub>2'''' and '''S<sub>N</sub>1'/S<sub>N</sub>i' substitutions'''). |
|||
In reaction conditions that favor a [[SN1 reaction|S<sub>N</sub>1 reaction]] mechanism the intermediate is a [[carbocation]] for which several [[resonance structure]]s are possible. This explains the product distribution (or '''product spread''') after recombination with [[nucleophile]] Y. This type of process is called an '''S<sub>N</sub>1' substitution'''. |
|||
==Scope== |
|||
Alternatively, it is possible for nucleophile to attack directly at the allylic position, displacing the leaving group in a single step, in a process referred to as '''S<sub>N</sub>2' substitution'''. This is likely in cases when the allyl compound is unhindered, and a strong [[nucleophile]] is used. The products will be similar to those seen with S<sub>N</sub>1' substitution. Thus reaction of 1-chloro-2-butene with [[sodium hydroxide]] gives a mixture of 2-buten-1-ol and 1-buten-3-ol: |
|||
Allylic shifts become the dominant reaction pathway when there is substantial resistance to a normal (non-allylic) substitution. For nucleophilic substitution, such resistance is known when there is substantial steric hindrance at or around the [[leaving group]], or if there is a [[geminal]] substituent destabilizing an accumulation of positive charge. The effects of substitution at the vinyl group are less clear.<ref>{{Citation |last=DeWolfe |first=Robert H. |title=Allylic reactions |date=1964-01-01 |work=The Alkenes: Vol. 1 (1964) |pages=690-691 |editor-last=Patai |editor-first=Saul |url=https://onlinelibrary.wiley.com/doi/10.1002/9780470771044.ch10 |access-date=2024-03-10 |place=Chichester, UK |publisher=John Wiley & Sons, Ltd. |language=en |doi=10.1002/9780470771044.ch10 |isbn=978-0-470-77104-4 |last2=Young |first2=William G.}}</ref> |
|||
Although rarer still than S<sub>N</sub>', allylic shifts can occur vinylogously, as a "butadienylic shift":<ref>''Molecular yardsticks. Synthesis of extended equilibrium transfer alkylating cross-link reagents and their use in the formation of macrocycles'' Stephen J. Brocchini, Martin Eberle, and Richard G. Lawton [[J. Am. Chem. Soc.]]; '''1988'''; 110(15) pp 5211 - 5212; {{doi|10.1021/ja00223a061}}</ref> |
|||
:CH<sub>3</sub>CH=CHCH<sub>2</sub>Cl {{unicode|→}} CH<sub>3</sub>CH=CHCH<sub>2</sub>OH + CH<sub>3</sub>CH(OH)CH=CH<sub>2</sub> |
|||
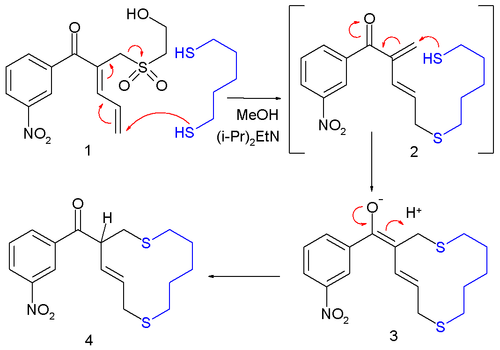
[[File:Lawton_reaction.png|alt=A macrocyclization: a 1,5-pentanedithiol terminus attacks the butadiene tail of a 1-substituted 2,4-pentadien-2-yl aryl ketone. Instead of forming an enol, the compound undergoes an allylic shift, expulsing the 1-substituent and leaving a 5-thioether 1,3-pentadien-2-yl ketone. The other end of the thiol then adds to the ketone in conjugate. |center|thumb|500x500px|MeOH is [[methanol]] solvent; (''i''{{Nbh}}Pr)<sub>2</sub>EtN is catalytic [[diisopropylethylamine]]]] |
|||
=== S<sub>N</sub>2' reduction === |
|||
| ⚫ | |||
In '''S<sub>N</sub>2' reduction''', a [[hydride]] allylically displaces a good [[leaving group]] in a formal [[organic reduction]], similar to the [[Whiting reaction|Whiting diene synthesis]]. One example occurred in [[taxol total synthesis]] (ring C):<ref>''Synthetic Studies on Taxol: Highly Stereoselective Construction of the Taxol C-Ring via S<sub>N</sub>2' Reduction of an Allylic Phosphonium Salt'' Masayuki Utsugi, Masayuki Miyano, and Masahisa Nakada [[Org. Lett.]]; '''2006'''; 8(14) pp 2973 - 2976; (Letter) {{doi|10.1021/ol0608606}}</ref> |
|||
:[[Image:SN2reduction.png|400px|S<sub>N</sub>2 reduction]] |
|||
Examples of allylic shifts: |
|||
| ⚫ | |||
The [[hydride]] is [[lithium aluminium hydride]] and the leaving group a [[phosphonium salt]]; the allylic shift causes the [[exocyclic]] double bond in the product. Only when the [[cyclohexane]] ring is properly substituted will the proton add ''[[trans isomer|trans]]'' to the adjacent [[methyl]] group. |
|||
=== Electrophilic allyl shifts === |
|||
{{reaction-stub}} |
|||
Allyl shifts can also take place with [[electrophile]]s. In the example below the [[carbonyl]] group in [[benzaldehyde]] is activated by [[diboronic acid]] prior to reaction with the allyl alcohol (see: [[Prins reaction]]):<ref>''Highly Selective and Robust Palladium-Catalyzed Carbon-Carbon Coupling between Allyl Alcohols and Aldehydes via Transient Allylboronic Acids'' Nicklas Selander, Sara Sebelius, Cesar Estay, Kálmán J. Szabó [[European Journal of Organic Chemistry]] Volume '''2006''', Issue 18 , Pages 4085 - 4087 {{doi|10.1002/ejoc.200600530}}</ref> |
|||
[[File:ElectrophilicAllylShift.png|alt=Electrophilic allyl shift|center|400x400px]] |
|||
The active catalyst system in this reaction is a combination of a [[palladium]] [[Molecular tweezer|pincer compound]] and [[P-Toluenesulfonic acid|''p''-toluenesulfonic acid]], the reaction product is obtained as a single [[regioisomer]] and [[stereoisomer]]. |
|||
== Examples == |
|||
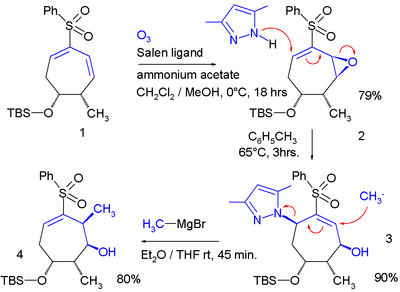
Repeated allylic shifts can "flip-flop" a double-bond between two possible locations:<ref>''Double Lawton S<sub>N</sub>2' Addition to Epoxyvinyl Sulfones: Selective Construction of the Stereotetrads of Aplyronine A'' Ahmad El-Awa and Philip Fuchs [[Org. Lett.]]; '''2006'''; 8(14) pp 2905 - 2908; (Letter) {{doi|10.1021/ol060530l}}</ref> |
|||
[[File:Double_Lawson_reaction.png|alt=A diene epoxide (from Jacobsen epoxidation) adds a pyrazole with an allylic shift. Then methylmagnesium bromide expulses the pyrazole with another allylic shift, returning the remaining double-bond to its original position. |center|400x400px]] |
|||
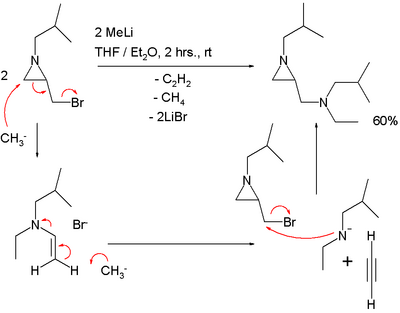
An S<sub>N</sub>2' reaction should explain the outcome of the reaction of an [[aziridine]] carrying a methylene bromide group with [[methyllithium]]:<ref>''Highly unusual conversion of 1-alkyl-2-(bromomethyl)aziridines into 1-alkyl-2-(''N''-alkyl-''N''-ethylaminomethyl)aziridines using methyllithium'' Matthias D'hooghe and Norbert De Kimpe [[Chem. Commun.]], '''2007''', 1275 - 1277, {{doi|10.1039/b616606g}}</ref> |
|||
:[[File:AziridineAllylicRearrangement.png|400x400px|conversion of 1-alkyl-2-(bromomethyl)aziridines into 1-alkyl-2-(''N''-alkyl-''N''-ethylaminomethyl)aziridines]] |
|||
In this reaction one equivalent of [[acetylene]] is lost. |
|||
=== Named reactions === |
|||
| ⚫ | |||
*[[Meyer–Schuster rearrangement]] |
|||
==References== |
|||
| ⚫ | |||
{{reflist}} |
|||
{{Organic reactions}} |
|||
| ⚫ | |||
[[Category:Reaction mechanisms]] |
|||
Latest revision as of 23:25, 10 March 2024
An allylic rearrangement or allylic shift is an organic chemical reaction in which reaction at a center vicinal to a double bond causes the double bond to shift to an adjacent pair of atoms:

It is encountered in both nucleophilic and electrophilic substitution, although it is usually suppressed relative to non-allylic substitution. For example, reaction of 1-chloro-2-butene with sodium hydroxide gives 2-buten-1-ol and 3-buten-2-ol:
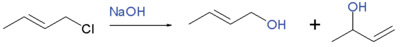
In the similar substitution of 1-chloro-3-methyl-2-butene, the secondary 2-methyl-3-buten-2-ol is produced in a yield of 85%, while that for the primary 3-methyl-2-buten-1-ol is 15%.
Allylic shifts occur because the transition state is an allyl intermediate. In other respects they are similar to classical nucleophilic substitution, and admit both bimolecular and monomolecular mechanisms (respectively the SN2' and SN1'/SNi' substitutions).
Scope
[edit]Allylic shifts become the dominant reaction pathway when there is substantial resistance to a normal (non-allylic) substitution. For nucleophilic substitution, such resistance is known when there is substantial steric hindrance at or around the leaving group, or if there is a geminal substituent destabilizing an accumulation of positive charge. The effects of substitution at the vinyl group are less clear.[1]
Although rarer still than SN', allylic shifts can occur vinylogously, as a "butadienylic shift":[2]
SN2' reduction
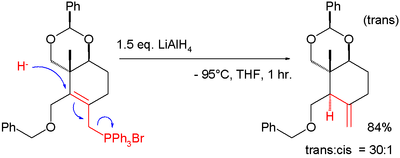
[edit]In SN2' reduction, a hydride allylically displaces a good leaving group in a formal organic reduction, similar to the Whiting diene synthesis. One example occurred in taxol total synthesis (ring C):[3]
The hydride is lithium aluminium hydride and the leaving group a phosphonium salt; the allylic shift causes the exocyclic double bond in the product. Only when the cyclohexane ring is properly substituted will the proton add trans to the adjacent methyl group.
Electrophilic allyl shifts
[edit]Allyl shifts can also take place with electrophiles. In the example below the carbonyl group in benzaldehyde is activated by diboronic acid prior to reaction with the allyl alcohol (see: Prins reaction):[4]
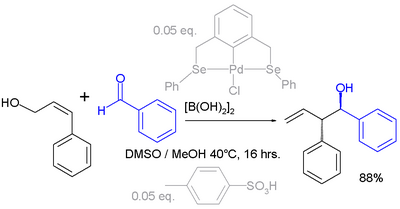
The active catalyst system in this reaction is a combination of a palladium pincer compound and p-toluenesulfonic acid, the reaction product is obtained as a single regioisomer and stereoisomer.
Examples
[edit]Repeated allylic shifts can "flip-flop" a double-bond between two possible locations:[5]
An SN2' reaction should explain the outcome of the reaction of an aziridine carrying a methylene bromide group with methyllithium:[6]
In this reaction one equivalent of acetylene is lost.
Named reactions
[edit]References
[edit]- ^ DeWolfe, Robert H.; Young, William G. (1964-01-01), Patai, Saul (ed.), "Allylic reactions", The Alkenes: Vol. 1 (1964), Chichester, UK: John Wiley & Sons, Ltd., pp. 690–691, doi:10.1002/9780470771044.ch10, ISBN 978-0-470-77104-4, retrieved 2024-03-10
- ^ Molecular yardsticks. Synthesis of extended equilibrium transfer alkylating cross-link reagents and their use in the formation of macrocycles Stephen J. Brocchini, Martin Eberle, and Richard G. Lawton J. Am. Chem. Soc.; 1988; 110(15) pp 5211 - 5212; doi:10.1021/ja00223a061
- ^ Synthetic Studies on Taxol: Highly Stereoselective Construction of the Taxol C-Ring via SN2' Reduction of an Allylic Phosphonium Salt Masayuki Utsugi, Masayuki Miyano, and Masahisa Nakada Org. Lett.; 2006; 8(14) pp 2973 - 2976; (Letter) doi:10.1021/ol0608606
- ^ Highly Selective and Robust Palladium-Catalyzed Carbon-Carbon Coupling between Allyl Alcohols and Aldehydes via Transient Allylboronic Acids Nicklas Selander, Sara Sebelius, Cesar Estay, Kálmán J. Szabó European Journal of Organic Chemistry Volume 2006, Issue 18 , Pages 4085 - 4087 doi:10.1002/ejoc.200600530
- ^ Double Lawton SN2' Addition to Epoxyvinyl Sulfones: Selective Construction of the Stereotetrads of Aplyronine A Ahmad El-Awa and Philip Fuchs Org. Lett.; 2006; 8(14) pp 2905 - 2908; (Letter) doi:10.1021/ol060530l
- ^ Highly unusual conversion of 1-alkyl-2-(bromomethyl)aziridines into 1-alkyl-2-(N-alkyl-N-ethylaminomethyl)aziridines using methyllithium Matthias D'hooghe and Norbert De Kimpe Chem. Commun., 2007, 1275 - 1277, doi:10.1039/b616606g