Isopentane: Difference between revisions
Nic.anfernee (talk | contribs) →Uses: Cryosectioning |
m neopentane (2,2-dimethylpropane) |
||
| (42 intermediate revisions by 30 users not shown) | |||
| Line 3: | Line 3: | ||
| Watchedfields = changed |
| Watchedfields = changed |
||
| verifiedrevid = 464398176 |
| verifiedrevid = 464398176 |
||
| ImageFile = Isopentane-2D-skeletal. |
| ImageFile = Isopentane-2D-skeletal.svg |
||
| ImageFile_Ref = {{chemboximage|correct|??}} |
| ImageFile_Ref = {{chemboximage|correct|??}} |
||
| ImageSize = 100 |
| ImageSize = 100 |
||
| Line 15: | Line 15: | ||
| ImageSize2 = 100 |
| ImageSize2 = 100 |
||
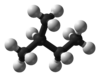
| ImageName2 = Ball and stick model of isopentane |
| ImageName2 = Ball and stick model of isopentane |
||
| PIN = 2-Methylbutane<ref name="IUPAC2013_652">{{cite book | title = Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book) | publisher = [[Royal Society of Chemistry|The Royal Society of Chemistry]] | date = 2014 | location = Cambridge | page = 652 | doi = 10.1039/9781849733069-FP001 | isbn = 978-0-85404-182-4 | quote = The names ‘isobutane’, ‘isopentane’ and ‘neopentane’ are no longer recommended.}}</ref> |
|||
| IUPACName = 2-Methylbutane<ref>{{Cite web|title=isopentane - Compound Summary|url=http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6556&loc=ec_rcs#x291|work=PubChem Compound|publisher=National Center for Biotechnology Information|accessdate=5 March 2012|location=USA|date=16 September 2004|at=Identification and Related Records}}</ref> |
|||
| OtherNames = Isopentane |
|||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| CASNo = 78-78-4 |
| CASNo = 78-78-4 |
||
| Line 39: | Line 40: | ||
}} |
}} |
||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| C |
| C=5 | H=12 |
||
| H = 12 |
|||
| Appearance = Colorless liquid |
| Appearance = Colorless liquid |
||
| Odor = |
| Odor = Gasoline-like |
||
| Density = 616 mg mL<sup>−1</sup><ref name="Wei">James Wei (1999), ''Molecular Symmetry, Rotational Entropy, and Elevated Melting Points''. Ind. Eng. Chem. Res., volume 38 issue 12, pp. 5019–5027 {{doi|10.1021/ie990588m}}</ref> |
| Density = 616 mg mL<sup>−1</sup><ref name="Wei">James Wei (1999), ''Molecular Symmetry, Rotational Entropy, and Elevated Melting Points''. Ind. Eng. Chem. Res., volume 38 issue 12, pp. 5019–5027 {{doi|10.1021/ie990588m}}</ref> |
||
| BoilingPtK = 300.9 to 301.3 |
| BoilingPtK = 300.9 to 301.3 |
||
| Line 50: | Line 50: | ||
| LambdaMax = 192 nm |
| LambdaMax = 192 nm |
||
| RefractIndex = 1.354 |
| RefractIndex = 1.354 |
||
| Viscosity = 0.214 cP (at 20 °C) |
|||
}} |
}} |
||
|Section4={{Chembox Thermochemistry |
|Section4={{Chembox Thermochemistry |
||
| DeltaHf = −179.1–−177.3 kJ mol<sup>−1</sup> |
| DeltaHf = −179.1–−177.3 kJ mol<sup>−1</sup> |
||
| DeltaHc = |
| DeltaHc = ~ 3.3 MJ mol<sup>−1</sup>, 19,664 Btu/lb |
||
| Entropy = 260.41 J K<sup>−1</sup> mol<sup>−1</sup> |
| Entropy = 260.41 J K<sup>−1</sup> mol<sup>−1</sup> |
||
| HeatCapacity = 164.85 J K<sup>−1</sup> mol<sup>−1</sup> |
| HeatCapacity = 164.85 J K<sup>−1</sup> mol<sup>−1</sup> |
||
| Line 60: | Line 61: | ||
| GHSPictograms = {{GHS flame}} {{GHS exclamation mark}} {{GHS health hazard}} {{GHS environment}} |
| GHSPictograms = {{GHS flame}} {{GHS exclamation mark}} {{GHS health hazard}} {{GHS environment}} |
||
| GHSSignalWord = '''DANGER''' |
| GHSSignalWord = '''DANGER''' |
||
| HPhrases = {{H-phrases|224| |
| HPhrases = {{H-phrases|224|301|302|305|336|411}} |
||
| PPhrases = {{P-phrases|210|261|273|301+310|331}} |
| PPhrases = {{P-phrases|210|261|273|301+310|331}} |
||
| EUIndex = 601-006-00-1 |
|||
| EUClass = {{Hazchem F+}} {{Hazchem Xn}} {{Hazchem N}} |
|||
| NFPA-H = 1 |
| NFPA-H = 1 |
||
| NFPA-F = 4 |
| NFPA-F = 4 |
||
| NFPA-R = 0 |
| NFPA-R = 0 |
||
| RPhrases = {{R12}}, {{R51/53}}, {{R65}}, {{R66}}, {{R67}} |
|||
| SPhrases = {{S2}}, {{S16}}, {{S29}}, {{S33}} |
|||
| FlashPtC = −51 |
| FlashPtC = −51 |
||
| AutoignitionPtC = 420 |
| AutoignitionPtC = 420 |
||
| Line 79: | Line 76: | ||
}} |
}} |
||
}} |
}} |
||
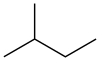
'''Isopentane''', also called '''methylbutane''' or '''2-methylbutane''', is a branched-chain saturated [[hydrocarbon]] (an [[alkane]]) with five [[carbon]] atoms, with formula {{chem|C|5|H|12}} or {{chem|CH(CH|3|)|2|(C|2|H|5|)}}. |
|||
'''Isopentane''', [[carbon|C<sub>5</sub>]][[hydrogen|H<sub>12</sub>]], also called '''methylbutane''' or '''2-methylbutane''', is a branched-chain [[alkane]] with five [[carbon]] atoms . Isopentane is an extremely [[Volatility (chemistry)|volatile]] and extremely [[flammable]] liquid at room [[temperature]] and [[pressure]]. The [[normal boiling point]] is just a few degrees above room temperature and isopentane will readily boil and evaporate away on a warm day. Isopentane is commonly used in conjunction with [[liquid nitrogen]] to achieve a liquid bath temperature of −160 °C. It is 1% or less of natural gas.<ref>Georg Hammer, Torsten Lübcke, Roland Kettner, Mark R. Pillarella, Herta Recknagel, Axel Commichau, Hans-Joachim Neumann and Barbara Paczynska-Lahme "Natural Gas" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. {{DOI|10.1002/14356007.a17_073.pub2}}</ref> |
|||
| ⚫ | |||
| ⚫ | |||
Isopentane is the name recommended by the [[International Union of Pure and Applied Chemistry]] (IUPAC).<ref>{{cite book | author=Panico, R.; & Powell, W. H. (Eds.) | title=A Guide to IUPAC Nomenclature of Organic Compounds 1993 | location=Oxford | publisher=Blackwell Science | year=1994 | isbn = 0-632-03488-2 | url = http://www.acdlabs.com/iupac/nomenclature/93/r93_679.htm}}</ref> An '''isopentyl''' group is a subset of the generic pentyl group. It has the chemical structure -CH<sub>2</sub>CH<sub>2</sub>CH(CH<sub>3</sub>)<sub>2</sub>. |
|||
Isopentane is commonly used in conjunction with [[liquid nitrogen]] to achieve a liquid bath temperature of −160 °C. [[Natural gas]] typically contains 1% or less isopentane,<ref>Georg Hammer, Torsten Lübcke, Roland Kettner, Mark R. Pillarella, Herta Recknagel, Axel Commichau, Hans-Joachim Neumann and Barbara Paczynska-Lahme "Natural Gas" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. {{doi|10.1002/14356007.a17_073.pub2}}</ref> but it is a significant component of [[natural gasoline]].<ref name=aver1958>Ivan F. Avery, L. V. Harvey (1958): ''[https://books.google.com/books?id=sC2JHAYfJb0C&pg=PA6 Natural-gasoline and Cycling Plants in the United States]'', Information circular, U.S. Department of the Interior, Bureau of Mines. 12 pages.</ref> |
|||
==Isomers== |
|||
| ⚫ | |||
| ⚫ | |||
The traditional name isopentane was still retained in the 1993 [[International Union of Pure and Applied Chemistry|IUPAC]] recommendations,<ref>[http://www.acdlabs.com/iupac/nomenclature/93/r93_679.htm Table 19(a) Acyclic and monocyclic hydrocarbons. Parent hydrocarbons]</ref><ref name="panico">{{cite book | editor=Panico, R. | editor2= Powell, W. H. | name-list-style= amp | title=A Guide to IUPAC Nomenclature of Organic Compounds 1993 | location=Oxford | publisher=Blackwell Science | year=1994 | isbn=0-632-03488-2}} |
|||
</ref> but is no longer recommended according to the 2013 recommendations.<ref name="IUPAC2013_652"/> The preferred IUPAC name is the systematic name 2-methylbutane. An '''isopentyl''' group is a subset of the generic pentyl group. It has the chemical structure -CH<sub>3</sub>CH<sub>2</sub>CH(CH<sub>3</sub>)<sub>2</sub>. |
|||
==Uses== |
==Uses== |
||
Isopentane is used in a closed loop in geothermal power production to drive turbines.<ref> |
Isopentane is used in a closed loop in geothermal power production to drive turbines.<ref>Byproduct Isopentane also used in some of the LPG plant to run the boiler and generate the power. |
||
| ⚫ | |||
Byproduct Isopentane also used in some of the LPG plant to run the boiler and generate the power. |
|||
| ⚫ | |||
Isopentane is used, in conjunction with [[dry ice]] or |
Isopentane is used, in conjunction with [[dry ice]] or liquid nitrogen, to freeze [[tissue (biology)|tissues]] for [[cryosection]]ing in [[histology]]. |
||
<ref>{{Cite web|url=http://www.uab.edu/research/administration/offices/ARP/ComparativePathology/Pathology/Histopathology/TissueSubmission/Pages/Freezing-Tissues-for-Cryosectioning.aspx|title = Animal Resources Program - the Office of the Vice President for Research | UAB}}</ref> |
|||
Isopentane is a major component (sometimes 30% or more) of natural gasoline, an analog of common [[petroleum]]-derived [[gasoline]] that is condensed from natural gas.<ref name=aver1958/> It has a substantially higher [[octane rating]] (RON 93.7) than ''n''-pentane (61.7), and therefore there is interest in conversion from the latter.<ref name=wang2014>Sheng Wang, Ying Zhang, Mao-Gang He, Xiong Zheng, and Li-Bin Chen (2014): "Thermal Diffusivity and Speed of Sound of Saturated Pentane from Light Scattering". ''International Journal of Thermophysics'', volume 35, pages 1450–1464. {{doi|10.1007/s10765-014-1718-x}}</ref> |
|||
==References== |
==References== |
||
| Line 104: | Line 105: | ||
{{Authority control}} |
{{Authority control}} |
||
[[Category:Alkanes]] |
[[Category:Alkanes]] |
||
Latest revision as of 13:57, 9 April 2024
| |

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylbutane[1] | |
| Other names
Isopentane
| |
| Identifiers | |
3D model (JSmol)
|
|
| 1730723 | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.039 |
| EC Number |
|
| 49318 | |
| MeSH | isopentane |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1265 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12 | |
| Molar mass | 72.151 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Gasoline-like |
| Density | 616 mg mL−1[2] |
| Melting point | −161 to −159 °C; −258 to −254 °F; 112 to 114 K |
| Boiling point | 27.8 to 28.2 °C; 81.9 to 82.7 °F; 300.9 to 301.3 K |
| Vapor pressure | 76.992 kPa (at 20 °C) |
Henry's law
constant (kH) |
7.2 nmol Pa−1 kg−1 |
| UV-vis (λmax) | 192 nm |
Refractive index (nD)
|
1.354 |
| Viscosity | 0.214 cP (at 20 °C) |
| Thermochemistry | |
Heat capacity (C)
|
164.85 J K−1 mol−1 |
Std molar
entropy (S⦵298) |
260.41 J K−1 mol−1 |
Std enthalpy of
formation (ΔfH⦵298) |
−179.1–−177.3 kJ mol−1 |
Std enthalpy of
combustion (ΔcH⦵298) |
~ 3.3 MJ mol−1, 19,664 Btu/lb |
| Hazards | |
| GHS labelling: | |
   
| |
| Danger | |
| H224, H301, H302, H305, H336, H411 | |
| P210, P261, P273, P301+P310, P331 | |
| NFPA 704 (fire diamond) | |
| Flash point | −51 °C (−60 °F; 222 K) |
| 420 °C (788 °F; 693 K) | |
| Explosive limits | 1.4–8.3% |
| Related compounds | |
Related alkanes
|
|
Related compounds
|
2-Ethyl-1-butanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Isopentane, also called methylbutane or 2-methylbutane, is a branched-chain saturated hydrocarbon (an alkane) with five carbon atoms, with formula C
5H
12 or CH(CH
3)
2(C
2H
5).
Isopentane is a volatile and flammable liquid. It is one of three structural isomers with the molecular formula C5H12, the others being pentane (n-pentane) and neopentane (2,2-dimethylpropane).
Isopentane is commonly used in conjunction with liquid nitrogen to achieve a liquid bath temperature of −160 °C. Natural gas typically contains 1% or less isopentane,[3] but it is a significant component of natural gasoline.[4]
Nomenclature
[edit]The traditional name isopentane was still retained in the 1993 IUPAC recommendations,[5][6] but is no longer recommended according to the 2013 recommendations.[1] The preferred IUPAC name is the systematic name 2-methylbutane. An isopentyl group is a subset of the generic pentyl group. It has the chemical structure -CH3CH2CH(CH3)2.
Uses
[edit]Isopentane is used in a closed loop in geothermal power production to drive turbines.[7]
Isopentane is used, in conjunction with dry ice or liquid nitrogen, to freeze tissues for cryosectioning in histology. [8]
Isopentane is a major component (sometimes 30% or more) of natural gasoline, an analog of common petroleum-derived gasoline that is condensed from natural gas.[4] It has a substantially higher octane rating (RON 93.7) than n-pentane (61.7), and therefore there is interest in conversion from the latter.[9]
References
[edit]- ^ a b Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 652. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The names 'isobutane', 'isopentane' and 'neopentane' are no longer recommended.
- ^ James Wei (1999), Molecular Symmetry, Rotational Entropy, and Elevated Melting Points. Ind. Eng. Chem. Res., volume 38 issue 12, pp. 5019–5027 doi:10.1021/ie990588m
- ^ Georg Hammer, Torsten Lübcke, Roland Kettner, Mark R. Pillarella, Herta Recknagel, Axel Commichau, Hans-Joachim Neumann and Barbara Paczynska-Lahme "Natural Gas" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_073.pub2
- ^ a b Ivan F. Avery, L. V. Harvey (1958): Natural-gasoline and Cycling Plants in the United States, Information circular, U.S. Department of the Interior, Bureau of Mines. 12 pages.
- ^ Table 19(a) Acyclic and monocyclic hydrocarbons. Parent hydrocarbons
- ^ Panico, R. & Powell, W. H., eds. (1994). A Guide to IUPAC Nomenclature of Organic Compounds 1993. Oxford: Blackwell Science. ISBN 0-632-03488-2.
- ^ Byproduct Isopentane also used in some of the LPG plant to run the boiler and generate the power. HS Orka HF Energy Plant IV Archived 2014-10-18 at the Wayback Machine
- ^ "Animal Resources Program - the Office of the Vice President for Research | UAB".
- ^ Sheng Wang, Ying Zhang, Mao-Gang He, Xiong Zheng, and Li-Bin Chen (2014): "Thermal Diffusivity and Speed of Sound of Saturated Pentane from Light Scattering". International Journal of Thermophysics, volume 35, pages 1450–1464. doi:10.1007/s10765-014-1718-x
External links
[edit]- International Chemical Safety Card 1153
- IUPAC Nomenclature of Organic Chemistry (online version of the "Blue Book")

