Glyceollin I: Difference between revisions
Appearance
Content deleted Content added
No edit summary |
Rescuing 0 sources and tagging 1 as dead.) #IABot (v2.0.9.5 |
||
| (23 intermediate revisions by 20 users not shown) | |||
| Line 1: | Line 1: | ||
{{Chembox |
{{Chembox |
||
| Verifiedfields = changed |
|||
| Watchedfields = changed |
| Watchedfields = changed |
||
| verifiedrevid = |
| verifiedrevid = 423639166 |
||
| ImageFile = Glyceollin I.svg |
| ImageFile = Glyceollin I.svg |
||
| |
| ImageSize = |
||
| |
| ImageAlt = |
||
| |
| PIN = (6a''S'',11a''S'')-2,2-Dimethyl-2''H'',6''H''-[1]benzofuro[3,2-''c'']pyrano[2,3-''h''][1]benzopyran-6a,9(11a''H'')-diol |
||
| OtherNames = (−)-Glyceollin I |
| OtherNames = (−)-Glyceollin I |
||
| |
|Section1={{Chembox Identifiers |
||
| CASNo_Ref = {{cascite|correct|??}} |
|||
| CASNo = 57103-57-8 |
|||
| |
| CASNo = 57103-57-8 |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| ⚫ | |||
| UNII = 6461TV6UCH |
|||
| ⚫ | |||
| PubChem = 162807 |
|||
| ⚫ | |||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} |
|||
| ⚫ | |||
| ChemSpiderID = 142931 |
|||
| ExactMass = 338.11542 u |
|||
| SMILES = CC1(C=Cc2c(ccc3c2OC[C@@]4([C@H]3Oc5c4ccc(c5)O)O)O1)C |
|||
| ⚫ | |||
| InChI = 1/C20H18O5/c1-19(2)8-7-12-15(25-19)6-4-13-17(12)23-10-20(22)14-5-3-11(21)9-16(14)24-18(13)20/h3-9,18,21-22H,10H2,1-2H3/t18-,20+/m0/s1 |
|||
| ⚫ | |||
| InChIKey = YIFYYPKWOQSCRI-AZUAARDMBS |
|||
| ⚫ | |||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
|||
| ⚫ | |||
| StdInChI = 1S/C20H18O5/c1-19(2)8-7-12-15(25-19)6-4-13-17(12)23-10-20(22)14-5-3-11(21)9-16(14)24-18(13)20/h3-9,18,21-22H,10H2,1-2H3/t18-,20+/m0/s1 |
|||
| Solubility = }} |
|||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
|||
| ⚫ | |||
| StdInChIKey = YIFYYPKWOQSCRI-AZUAARDMSA-N |
|||
| ⚫ | |||
| |
| RTECS = |
||
| |
| MeSHName = |
||
| ChEBI_Ref = {{ebicite|changed|EBI}} |
|||
| ChEBI = 16470 |
|||
| KEGG_Ref = {{keggcite|changed|kegg}} |
|||
| KEGG = C01701 }} |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| FlashPt = |
|||
| AutoignitionPt = }} |
|||
}} |
}} |
||
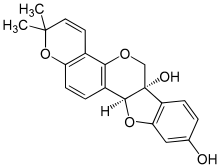
'''Glyceollin I''' is a [[glyceollin]], a type of [[prenylated]] [[pterocarpan]]. It is a [[phytoalexin]] found in the [[soybean]].<ref> |
'''Glyceollin I''' is a [[glyceollin]], a type of [[prenylated]] [[pterocarpan]]. It is a [[phytoalexin]] found in the [[soybean]].<ref>{{Cite journal | last1 = Zimmermann | first1 = M. C. | last2 = Tilghman | first2 = S. L. | last3 = Boué | first3 = S. M. | last4 = Salvo | first4 = V. A. | last5 = Elliott | first5 = S. | last6 = Williams | first6 = K. Y. | last7 = Skripnikova | first7 = E. V. | last8 = Ashe | first8 = H. | last9 = Payton-Stewart | first9 = F. | doi = 10.1124/jpet.109.160382 | last10 = Vanhoy-Rhodes | first10 = L. | last11 = Fonseca | first11 = J. P. | last12 = Corbitt | first12 = C. | last13 = Collins-Burow | first13 = B. M. | last14 = Howell | first14 = M. H. | last15 = Lacey | first15 = M. | last16 = Shih | first16 = B. Y. | last17 = Carter-Wientjes | first17 = C. | last18 = Cleveland | first18 = T. E. | last19 = McLachlan | first19 = J. A. | last20 = Wiese | first20 = T. E. | last21 = Beckman | first21 = B. S. | last22 = Burow | first22 = M. E. | title = Glyceollin I, a Novel Antiestrogenic Phytoalexin Isolated from Activated Soy | journal = Journal of Pharmacology and Experimental Therapeutics | volume = 332 | issue = 1 | pages = 35–45 | year = 2009 | pmid = 19797619| pmc =2802480 }}</ref> |
||
[[Glyceollin synthase]] is an enzyme responsible for the production of glyceollin.<ref>{{ |
[[Glyceollin synthase]] is an enzyme responsible for the production of glyceollin.<ref>{{Cite journal | last1 = Welle | first1 = R. | last2 = Grisebach | first2 = H. | doi = 10.1016/0003-9861(88)90627-3 | title = Induction of phytoalexin synthesis in soybean: Enzymatic cyclization of prenylated pterocarpans to glyceollin isomers | journal = Archives of Biochemistry and Biophysics | volume = 263 | issue = 1 | pages = 191–198 | year = 1988 | pmid = 3369863}}</ref> The five substrates of this enzyme are 2-dimethylallyl-(6aS,11aS)-3,6a,9-trihydroxypterocarpan, 4-dimethylallyl-(6aS,11aS)-3,6a,9-trihydroxypterocarpan, [[nicotinamide adenine dinucleotide phosphate|NADPH]], [[hydrogen ion|H<sup>+</sup>]], and [[oxygen|O<sub>2</sub>]], whereas its three products are glyceollin, [[nicotinamide adenine dinucleotide phosphate|NADP<sup>+</sup>]], and [[water|H<sub>2</sub>O]]. |
||
In in vitro studies, this molecule has been shown to exhibit [[antiestrogenic]] properties.<ref>{{Cite journal | last1 = Payton-Stewart | first1 = F. | last2 = Khupse | first2 = R. S. | last3 = Boué | first3 = S. M. | last4 = Elliott | first4 = S. | last5 = Zimmermann | first5 = M. C. | last6 = Skripnikova | first6 = E. V. | last7 = Ashe | first7 = H. | last8 = Tilghman | first8 = S. L. | last9 = Beckman | first9 = B. S. | doi = 10.1016/j.steroids.2010.05.007 | last10 = Cleveland | first10 = T. E. | last11 = McLachlan | first11 = J. A. | last12 = Bhatnagar | first12 = D. | last13 = Wiese | first13 = T. E. | last14 = Erhardt | first14 = P. | last15 = Burow | first15 = M. E. | title = Glyceollin I enantiomers distinctly regulate ER-mediated gene expression | journal = Steroids | volume = 75 | issue = 12 | pages = 870–878 | year = 2010 | pmid = 20493896 | s2cid = 14878980 | url = https://naldc-legacy.nal.usda.gov/naldc/download.xhtml?id=44156&content=PDF | hdl = 11336/58472 | hdl-access = free }}{{Dead link|date=June 2024 |bot=InternetArchiveBot |fix-attempted=yes }}</ref> |
|||
This molecule exhibits [[antiestrogenic]] properties.<ref>Glyceollin I enantiomers distinctly regulate ER-mediated gene expression. Florastina Payton-Stewart, Rahul S. Khupse, Stephen M. Boué, Steven Elliott, M. Carla Zimmermann, Elena V. Skripnikova, Hasina Ashe, Syreeta L. Tilghman, Barbara S. Beckman, Thomas E. Cleveland, John A. McLachlan, Deepak Bhatnagar, Thomas E. Wiese, Paul Erhardt and Matthew E. Burow, Steroids, Volume 75, Issue 12, December 2010, Pages 870-878, {{doi|10.1016/j.steroids.2010.05.007}}, {{PMID|20493896}}</ref> |
|||
==References== |
== References == |
||
{{ |
{{Reflist}} |
||
<!-- ==External links== --> |
<!-- ==External links== --> |
||
{{Pterocarpan}} |
{{Pterocarpan}} |
||
{{Estrogen receptor modulators}} |
|||
[[Category:Antiestrogens]] |
|||
[[Category:Pterocarpans]] |
[[Category:Pterocarpans]] |
||
[[Category:Phytoalexins]] |
|||
[[Category:Heterocyclic compounds with 5 rings]] |
|||
{{ |
{{aromatic-stub}} |
||
Latest revision as of 19:15, 8 June 2024
| |
| Names | |
|---|---|
| Preferred IUPAC name
(6aS,11aS)-2,2-Dimethyl-2H,6H-[1]benzofuro[3,2-c]pyrano[2,3-h][1]benzopyran-6a,9(11aH)-diol | |
| Other names
(−)-Glyceollin I
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.222.666 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H18O5 | |
| Molar mass | 338 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Glyceollin I is a glyceollin, a type of prenylated pterocarpan. It is a phytoalexin found in the soybean.[1]
Glyceollin synthase is an enzyme responsible for the production of glyceollin.[2] The five substrates of this enzyme are 2-dimethylallyl-(6aS,11aS)-3,6a,9-trihydroxypterocarpan, 4-dimethylallyl-(6aS,11aS)-3,6a,9-trihydroxypterocarpan, NADPH, H+, and O2, whereas its three products are glyceollin, NADP+, and H2O.
In in vitro studies, this molecule has been shown to exhibit antiestrogenic properties.[3]
References
[edit]- ^ Zimmermann, M. C.; Tilghman, S. L.; Boué, S. M.; Salvo, V. A.; Elliott, S.; Williams, K. Y.; Skripnikova, E. V.; Ashe, H.; Payton-Stewart, F.; Vanhoy-Rhodes, L.; Fonseca, J. P.; Corbitt, C.; Collins-Burow, B. M.; Howell, M. H.; Lacey, M.; Shih, B. Y.; Carter-Wientjes, C.; Cleveland, T. E.; McLachlan, J. A.; Wiese, T. E.; Beckman, B. S.; Burow, M. E. (2009). "Glyceollin I, a Novel Antiestrogenic Phytoalexin Isolated from Activated Soy". Journal of Pharmacology and Experimental Therapeutics. 332 (1): 35–45. doi:10.1124/jpet.109.160382. PMC 2802480. PMID 19797619.
- ^ Welle, R.; Grisebach, H. (1988). "Induction of phytoalexin synthesis in soybean: Enzymatic cyclization of prenylated pterocarpans to glyceollin isomers". Archives of Biochemistry and Biophysics. 263 (1): 191–198. doi:10.1016/0003-9861(88)90627-3. PMID 3369863.
- ^ Payton-Stewart, F.; Khupse, R. S.; Boué, S. M.; Elliott, S.; Zimmermann, M. C.; Skripnikova, E. V.; Ashe, H.; Tilghman, S. L.; Beckman, B. S.; Cleveland, T. E.; McLachlan, J. A.; Bhatnagar, D.; Wiese, T. E.; Erhardt, P.; Burow, M. E. (2010). "Glyceollin I enantiomers distinctly regulate ER-mediated gene expression". Steroids. 75 (12): 870–878. doi:10.1016/j.steroids.2010.05.007. hdl:11336/58472. PMID 20493896. S2CID 14878980.[permanent dead link]
