Lanthanum aluminate: Difference between revisions
Ozzie10aaaa (talk | contribs) |
spam Undid revision 1225269521 by 192.76.8.79 (talk) |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 34: | Line 34: | ||
===Epitaxial thin films=== |
===Epitaxial thin films=== |
||
[[Epitaxial growth|Epitaxially grown]] [[thin films]] of LAO can serve various purposes for [[electron correlation|correlated electrons]] heterostructures and devices. LAO is sometimes used as an epitaxial insulator between two conductive layers. Epitaxial LAO films can be grown by several methods, most commonly by [[pulsed laser deposition]] (PLD) and [[molecular beam epitaxy]] (MBE).{{cn}} |
[[Epitaxial growth|Epitaxially grown]] [[thin films]] of LAO can serve various purposes for [[electron correlation|correlated electrons]] heterostructures and devices. LAO is sometimes used as an epitaxial insulator between two conductive layers. Epitaxial LAO films can be grown by several methods, most commonly by [[pulsed laser deposition]] (PLD) and [[molecular beam epitaxy]] (MBE).{{cn|date=September 2023}} |
||
[[Image:LAOSTO Interface.png|thumb|A schematic cross-section of the [[2DEG]] formed at [[Lanthanum aluminate-strontium titanate interface|LAO-STO interfaces]]]] |
[[Image:LAOSTO Interface.png|thumb|A schematic cross-section of the [[2DEG]] formed at [[Lanthanum aluminate-strontium titanate interface|LAO-STO interfaces]]]] |
||
Latest revision as of 04:10, 11 June 2024
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.031.290 |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| LaAlO3 | |
| Molar mass | 213.89 g/mol |
| Appearance | optically transparent, tan to brown |
| Odor | odorless |
| Density | 6.52 g/cm3 |
| Melting point | 2,080 °C (3,780 °F; 2,350 K) |
| Insoluble in mineral acids at 25 °C. Soluble in H3PO3 > 150 °C[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lanthanum aluminate is an inorganic compound with the formula LaAlO3, often abbreviated as LAO. It is an optically transparent ceramic oxide with a distorted perovskite structure.
Properties
[edit]Crystalline LaAlO3 has a relatively high relative dielectric constant of ~25. LAO's crystal structure is a rhombohedral distorted perovskite with a pseudocubic lattice parameter of 3.787 angstroms at room temperature[2] (although one source claims the lattice parameter is 3.82[3]). Polished single crystal LAO surfaces show twin defects visible to the naked eye.
Uses
[edit]Epitaxial thin films
[edit]Epitaxially grown thin films of LAO can serve various purposes for correlated electrons heterostructures and devices. LAO is sometimes used as an epitaxial insulator between two conductive layers. Epitaxial LAO films can be grown by several methods, most commonly by pulsed laser deposition (PLD) and molecular beam epitaxy (MBE).[citation needed]
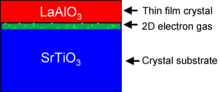
LAO-STO interfaces
The most important and common use for epitaxial LAO is at the lanthanum aluminate-strontium titanate interface. In 2004, it was discovered that when 4 or more unit cells of LAO are epitaxially grown on strontium titanate (SrTiO3, STO), a conductive 2-dimensional layer is formed at their interface.[4] Individually, LaAlO3 and SrTiO3 are non-magnetic insulators, yet LaAlO3/SrTiO3 interfaces exhibit electrical conductivity,[4] superconductivity,[5] ferromagnetism,[6] large negative in-plane magnetoresistance,[7] and giant persistent photoconductivity.[8] The study of how these properties emerge at the LaAlO3/SrTiO3 interface is a growing area of research in condensed matter physics.
Substrates
[edit]Single crystals of lanthanum aluminate are commercially available as a substrate for the epitaxial growth of perovskites,[1][9] and particularly for cuprate superconductors.
Non-epitaxial thin films
[edit]Thin films of lanthanum aluminate were considered as candidate materials for high-k dielectrics in the early-mid 2000s. Despite their attractive relative dielectric constant of ~25, they were not stable enough in contact with silicon at the relevant temperatures (~1000 °C).[10]
See also
[edit]- Lanthanum aluminate-strontium titanate interface
- High-k dielectrics
- LSAT (oxide)
- Perovskite structure
References
[edit]- ^ a b LaAlO3 specifications from the supplier MTI Corp. Archived 2013-11-01 at the Wayback Machine
- ^ "LaAlO3". MTI Corp. Retrieved 4 August 2015.
- ^ "LaAlO3". Crystec. Retrieved 3 August 2015.
- ^ a b Ohtomo; Hwang (29 Jan 2004). "A high-mobility electron gas at the LaAlO3/SrTiO3 heterointerface". Nature. 427 (6973): 423–6. Bibcode:2004Natur.427..423O. doi:10.1038/nature02308. PMID 14749825. S2CID 4419873.
- ^ Gariglio, S; Reyren, N; Caviglia, A D; Triscone, J-M (2009). "Superconductivity at the LaAlO3/SrTiO3 interface" (PDF). Journal of Physics: Condensed Matter. 21 (16): 164213. Bibcode:2009JPCM...21p4213G. doi:10.1088/0953-8984/21/16/164213. ISSN 0953-8984. PMID 21825393. S2CID 41420637.
- ^ Bert; Kalisky, Bell; Kim, Hikita; Hwang, Moler (4 September 2011). "Direct imaging of the coexistence of ferromagnetism and superconductivity at the LaAlO3/SrTiO3 interface". Nature Physics. 7 (10): 767. arXiv:1108.3150. Bibcode:2011NatPh...7..767B. doi:10.1038/nphys2079. S2CID 10809252.
- ^ Ben Shalom; Sachs, Rakhmilevitch; Palevski, Dagan (26 March 2010). "Tuning Spin-Orbit Coupling and Superconductivity at the SrTiO3/LaAlO3 Interface: A Magnetotransport Study". Physical Review Letters. 104 (12): 126802. arXiv:1001.0781. Bibcode:2010PhRvL.104l6802B. doi:10.1103/PhysRevLett.104.126802. PMID 20366556. S2CID 43174779.
- ^ Tebano, Antonello; E Fabbri; D Pergolesi; G Balestrino; E Traversa (19 January 2012). "Room-Temperature Giant Persistent Photoconductivity in SrTiO3/LaAlO3 Heterostructures". ACS Nano. 6 (2): 1278–1283. doi:10.1021/nn203991q. PMID 22260261.
- ^ LaAlO3 specifications from the supplier SurfaceNet
- ^ P. Sivasubramani; et al. (2005). "Outdiffusion of La and Al from amorphous LaAlO3 in direct contact with Si (001)" (PDF). Applied Physics Letters. 86 (20): 201901. Bibcode:2005ApPhL..86t1901S. doi:10.1063/1.1928316.
