Chlorobis(cyclooctene)rhodium dimer: Difference between revisions
m Chembox: rm/replace deprecated params. Fix unknown parameters (via AWB script) |
m Moving Category:Dimerization (chemistry) to Category:Dimers (chemistry) per Wikipedia:Categories for discussion/Speedy |
||
| (16 intermediate revisions by 9 users not shown) | |||
| Line 1: | Line 1: | ||
{{Chembox |
{{Chembox |
||
| |
|ImageFile = Rh2Cl2(coe)4corrected.png |
||
| ImageSize = |
|||
| ImageAlt = |
|||
| IUPACName = |
|||
| OtherNames = |
|||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| |
|CASNo = 12279-09-3 |
||
| |
|PubChem = 53384308 |
||
|ChemSpiderID = 21613963 |
|||
| SMILES = }} |
|||
|InChI=1S/4C8H14.2ClH.2Rh/c4*1-2-4-6-8-7-5-3-1;;;;/h4*1-2H,3-8H2;2*1H;;/p-2/b4*2-1-;;;; |
|||
|InChIKey = ZFCBAJWXKUDJSW-XFCUKONHSA-L |
|||
|SMILES = C1CCCC=CCC1.C1CCCC=CCC1.C1CCCC=CCC1.C1CCCC=CCC1.[Cl-].[Cl-].[Rh].[Rh] }} |
|||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| |
|Formula = C<sub>32</sub>H<sub>56</sub>Cl<sub>2</sub>Rh<sub>2</sub> |
||
| |
|MolarMass = 717.50 |
||
| |
|Appearance = red-brown solid |
||
}} |
|||
| Density = |
|||
| MeltingPt = |
|||
| BoilingPt = |
|||
| Solubility = }} |
|||
|Section3={{Chembox Hazards |
|Section3={{Chembox Hazards |
||
|GHSPictograms = {{GHS07}} |
|||
| MainHazards = |
|||
|GHSSignalWord = Warning |
|||
| FlashPt = |
|||
|HPhrases = {{H-phrases|302|312|315|319|332|335}} |
|||
| AutoignitionPt = }} |
|||
|PPhrases = {{P-phrases|261|264|270|271|280|301+312|302+352|304+312|304+340|305+351+338|312|321|322|330|332+313|337+313|362|363|403+233|405|501}} |
|||
}} |
|||
}} |
}} |
||
'''Chlorobis(cyclooctene)rhodium dimer''' is an [[organorhodium compound]] with the formula Rh<sub>2</sub>Cl<sub>2</sub>(C<sub>8</sub>H<sub>14</sub>)<sub>4</sub>, where C<sub>8</sub>H<sub>14</sub> is ''cis''-[[cyclooctene]]. Sometimes abbreviated Rh<sub>2</sub>Cl<sub>2</sub>(coe)<sub>4</sub>, it is a red-brown, air-sensitive solid that is a precursor to many other organorhodium compounds and catalysts. |
'''Chlorobis(cyclooctene)rhodium dimer''' is an [[organorhodium compound]] with the formula Rh<sub>2</sub>Cl<sub>2</sub>(C<sub>8</sub>H<sub>14</sub>)<sub>4</sub>, where C<sub>8</sub>H<sub>14</sub> is ''cis''-[[cyclooctene]]. Sometimes abbreviated Rh<sub>2</sub>Cl<sub>2</sub>(coe)<sub>4</sub>, it is a red-brown, air-sensitive solid that is a precursor to many other organorhodium compounds and catalysts. |
||
The complex is prepared by treating an alcohol solution of [[water of crystallization|hydrated]] [[rhodium trichloride]] with cyclooctene at room temperature.<ref>Van der Ent, A.; Onderdelinden, A. L. "Chlorobis(cyclooctene)rhodium(I) and di-μ-chlorobis[bis(cryclooctene)iridium] (I) complexes" Inorganic Syntheses 1973, volume 14, pp. 92-5. {{ |
The complex is prepared by treating an alcohol solution of [[water of crystallization|hydrated]] [[rhodium trichloride]] with cyclooctene at room temperature.<ref>Van der Ent, A.; Onderdelinden, A. L. "Chlorobis(cyclooctene)rhodium(I) and di-μ-chlorobis[bis(cryclooctene)iridium] (I) complexes" Inorganic Syntheses 1973, volume 14, pp. 92-5. {{doi|10.1002/9780470132456.ch18}}</ref> The coe ligands are easily displaced by other more basic [[ligand]]s, more so than the diene ligands in the related complex [[cyclooctadiene rhodium chloride dimer]]. |
||
==Catalyst for C-H activation== |
|||
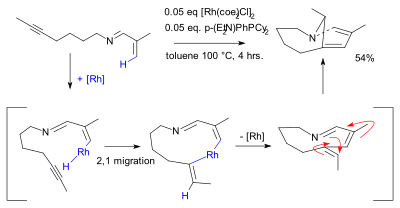
[[C-H activation]] is often catalyzed by chlorobis(cyclooctene)rhodium dimer as demonstrated in the synthesis of a strained bicyclic enamine.<ref>{{cite journal | last1 = Yotphan | first1 = Sirilata | last2 = Bergman | first2 = Robert G. | last3 = Ellman | first3 = Jonathan A. | title = ''The Stereoselective Formation of Bicyclic Enamines with Bridgehead Unsaturation via Tandem C–H Bond Activation/Alkenylation/ Electrocyclization'' | journal = [[J. Am. Chem. Soc.]] | date = 2008 | volume = 130 | issue = 8| pages = 2452–2453 | doi = 10.1021/ja710981b | pmid = 18247623 | pmc = 3062933 }}</ref> |
|||
:[[Image:BergMannCHActivation2008.svg|400px|center|C–H bond activation Yotphan 2008]] |
|||
The synthesis of a mescaline analogue involves enantioselective annulation of an aryl imine via a C-H activation.<ref>{{Cite journal|title = Synthesis of a Tricyclic Mescaline Analogue by Catalytic C−H Bond Activation|journal = Organic Letters|date = 2003-04-01|issn = 1523-7060|pages = 1301–1303|volume = 5|issue = 8|doi = 10.1021/ol034228d|first1 = Kateri A.|last1 = Ahrendt|first2 = Robert G.|last2 = Bergman|first3 = Jonathan A.|last3 = Ellman|pmid=12688744}}</ref> |
|||
[[File:Mescalineprep.jpg|centre|frameless|482x482px]] |
|||
The total synthesis of lithospermic acid employs "guided C-H functionalization" late stage to a highly functionalized system. The directing group, a [[Chirality (chemistry)|chiral]] nonracemic imine, is capable of performing an intramolecular alkylation, which allows for the rhodium-catalyzed conversion of imine to the dihydrobenzofuran.<ref>{{cite journal | title = Total Synthesis of (+)-Lithospermic Acid by Asymmetric Intramolecular Alkylation via Catalytic C-H Bond Activation | journal = J. Am. Chem. Soc. | year = 2005 | volume = 127 | issue = 39 | pages = 13496–13497 | last1 = O'Malley | first1 = S. J. | last2 = Tan | first2 = K. L. | last3 = Watzke | first3 = A. | last4 = Bergman | first4 = R. G. | last5 = Ellman | first5 = J. A. | doi = 10.1021/ja052680h | pmid=16190703}}</ref> |
|||
[[File:Natural Product Synth Ellman Figure 1.png|750px|center|Key step in synthesis of lithospermic acid]] |
|||
==References== |
==References== |
||
<references /> |
<references /> |
||
| Line 32: | Line 38: | ||
{{Rhodium compounds}} |
{{Rhodium compounds}} |
||
[[Category: |
[[Category:Organorhodium compounds]] |
||
[[Category:Homogeneous catalysis]] |
[[Category:Homogeneous catalysis]] |
||
[[Category:Alkene complexes]] |
[[Category:Alkene complexes]] |
||
[[Category:Dimers (chemistry)]] |
[[Category:Dimers (chemistry)]] |
||
[[Category:Chloro complexes]] |
|||
[[Category:Rhodium(I) compounds]] |
|||
Latest revision as of 17:17, 18 June 2024

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.152.028 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C32H56Cl2Rh2 | |
| Molar mass | 717.50 |
| Appearance | red-brown solid |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H312, H315, H319, H332, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chlorobis(cyclooctene)rhodium dimer is an organorhodium compound with the formula Rh2Cl2(C8H14)4, where C8H14 is cis-cyclooctene. Sometimes abbreviated Rh2Cl2(coe)4, it is a red-brown, air-sensitive solid that is a precursor to many other organorhodium compounds and catalysts.
The complex is prepared by treating an alcohol solution of hydrated rhodium trichloride with cyclooctene at room temperature.[1] The coe ligands are easily displaced by other more basic ligands, more so than the diene ligands in the related complex cyclooctadiene rhodium chloride dimer.
Catalyst for C-H activation
[edit]C-H activation is often catalyzed by chlorobis(cyclooctene)rhodium dimer as demonstrated in the synthesis of a strained bicyclic enamine.[2]
The synthesis of a mescaline analogue involves enantioselective annulation of an aryl imine via a C-H activation.[3]

The total synthesis of lithospermic acid employs "guided C-H functionalization" late stage to a highly functionalized system. The directing group, a chiral nonracemic imine, is capable of performing an intramolecular alkylation, which allows for the rhodium-catalyzed conversion of imine to the dihydrobenzofuran.[4]

References
[edit]- ^ Van der Ent, A.; Onderdelinden, A. L. "Chlorobis(cyclooctene)rhodium(I) and di-μ-chlorobis[bis(cryclooctene)iridium] (I) complexes" Inorganic Syntheses 1973, volume 14, pp. 92-5. doi:10.1002/9780470132456.ch18
- ^ Yotphan, Sirilata; Bergman, Robert G.; Ellman, Jonathan A. (2008). "The Stereoselective Formation of Bicyclic Enamines with Bridgehead Unsaturation via Tandem C–H Bond Activation/Alkenylation/ Electrocyclization". J. Am. Chem. Soc. 130 (8): 2452–2453. doi:10.1021/ja710981b. PMC 3062933. PMID 18247623.
- ^ Ahrendt, Kateri A.; Bergman, Robert G.; Ellman, Jonathan A. (2003-04-01). "Synthesis of a Tricyclic Mescaline Analogue by Catalytic C−H Bond Activation". Organic Letters. 5 (8): 1301–1303. doi:10.1021/ol034228d. ISSN 1523-7060. PMID 12688744.
- ^ O'Malley, S. J.; Tan, K. L.; Watzke, A.; Bergman, R. G.; Ellman, J. A. (2005). "Total Synthesis of (+)-Lithospermic Acid by Asymmetric Intramolecular Alkylation via Catalytic C-H Bond Activation". J. Am. Chem. Soc. 127 (39): 13496–13497. doi:10.1021/ja052680h. PMID 16190703.