Thiethylperazine: Difference between revisions
Undid revision 659302786 by 89.243.67.93 (talk) remove content added by sock of blocked user Nuklear |
removed Category:Piperazines; added Category:4-Methylpiperazin-1-yl compounds using HotCat |
||
| (31 intermediate revisions by 19 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical compound}} |
|||
{{Drugbox |
{{Drugbox |
||
| verifiedrevid = 470606662 |
| verifiedrevid = 470606662 |
||
| IUPAC_name = 2-(ethylthio)-10-[3-(4-methylpiperazin-1-yl)propyl]-10''H''-phenothiazine |
| IUPAC_name = 2-(ethylthio)-10-[3-(4-methylpiperazin-1-yl)propyl]-10''H''-phenothiazine |
||
| image = Thiethylperazine |
| image = Thiethylperazine.svg |
||
<!--Clinical data-->| tradename = Torecan, Norzine |
|||
| Drugs.com = {{drugs.com|CONS|thiethylperazine}} |
|||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|||
| pregnancy_US = <!-- A / B / C / D / X --> |
|||
| pregnancy_category = |
|||
| legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> |
|||
| legal_UK = <!-- GSL / P / POM / CD --> |
|||
| legal_US = <!-- OTC / Rx-only --> |
|||
| legal_status = |
|||
| routes_of_administration = <!--Pharmacokinetic data--> |
|||
| bioavailability = |
|||
| protein_bound = 60% |
|||
| metabolism = |
|||
| elimination_half-life = |
|||
| excretion = <!--Identifiers--> |
|||
| IUPHAR_ligand = 7306 |
|||
| CAS_number_Ref = {{cascite|correct|??}} |
|||
| CAS_number = 1420-55-9 |
|||
| ATC_prefix = R06 |
|||
| ATC_suffix = AD03 |
|||
| ATC_supplemental = |
|||
| PubChem = 5440 |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| DrugBank = DB00372 |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| ChemSpiderID = 5245 |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = 8ETK1WAF6R |
|||
| KEGG_Ref = {{keggcite|correct|kegg}} |
|||
| KEGG = D02354 |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
| ChEBI = 9544 |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
| ChEMBL = 1378 |
|||
<!--Chemical data-->| C = 22 |
|||
| H = 29 |
|||
| N = 3 |
|||
| S = 2 |
|||
| smiles = S(c2cc1N(c3c(Sc1cc2)cccc3)CCCN4CCN(C)CC4)CC |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChI = 1S/C22H29N3S2/c1-3-26-18-9-10-22-20(17-18)25(19-7-4-5-8-21(19)27-22)12-6-11-24-15-13-23(2)14-16-24/h4-5,7-10,17H,3,6,11-16H2,1-2H3 |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = XCTYLCDETUVOIP-UHFFFAOYSA-N |
|||
}} |
|||
'''Thiethylperazine''' ('''Torecan''', '''Norzine''') is an [[antiemetic]]<ref>{{cite journal | vauthors = Tamboline BL, Mcgillivray DC, Bogoch A | title = The Effects of Thiethylperazine Dimaleate (Torecan) on Nausea and Vomiting | journal = Canadian Medical Association Journal | volume = 92 | issue = 8 | pages = 422–423 | date = February 1965 | pmid = 14261157 | pmc = 1928133 }}</ref> of the [[phenothiazine]] class. It is an antagonist of [[dopamine receptor]]s ([[Dopamine receptor D1|DRD1]], [[Dopamine receptor D2|DRD2]], [[Dopamine receptor D4|DRD4]]) as well as of [[5-HT2A receptor|5-HT<sub>2A</sub>]], [[5-HT2C receptor|5-HT<sub>2C</sub>]] receptors, [[Muscarinic acetylcholine receptor|mAChRs]] (1 through 5), [[Alpha-1 adrenergic receptor|α<sub>1</sub> adrenergic receptor]] and [[Histamine H1 receptor|H<sub>1</sub> receptor]]. |
|||
<!--Clinical data--> |
|||
| tradename = |
|||
| Drugs.com = {{drugs.com|CONS|thiethylperazine}} |
|||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|||
| pregnancy_US = <!-- A / B / C / D / X --> |
|||
| pregnancy_category = |
|||
| legal_AU = <!-- Unscheduled / S2 / S4 / S8 --> |
|||
| legal_UK = <!-- GSL / P / POM / CD --> |
|||
| legal_US = <!-- OTC / Rx-only --> |
|||
| legal_status = |
|||
| routes_of_administration = |
|||
Thiethylperazine activates the transport protein [[ABCC1]] that clears [[beta-amyloid]] from brains of mice.<ref name="pmid21881209">{{cite journal | vauthors = Krohn M, Lange C, Hofrichter J, Scheffler K, Stenzel J, Steffen J, Schumacher T, Brüning T, Plath AS, Alfen F, Schmidt A, Winter F, Rateitschak K, Wree A, Gsponer J, Walker LC, Pahnke J | display-authors = 6 | title = Cerebral amyloid-β proteostasis is regulated by the membrane transport protein ABCC1 in mice | journal = The Journal of Clinical Investigation | volume = 121 | issue = 10 | pages = 3924–3931 | date = October 2011 | pmid = 21881209 | pmc = 3195473 | doi = 10.1172/JCI57867 }} |
|||
<!--Pharmacokinetic data--> |
|||
* {{cite press release |date=September 1, 2011 |title=Alzheimer disease: Transport protein ABCC1 plays key role in clearing beta-amyloid from brains of mice |website=ScienceDaily |url=https://www.sciencedaily.com/releases/2011/09/110901134628.htm}}</ref> |
|||
| bioavailability = |
|||
| protein_bound = 60% |
|||
| metabolism = |
|||
| elimination_half-life = |
|||
| excretion = |
|||
== Pharmacokinetics == |
|||
<!--Identifiers--> |
|||
<ref>{{cite web |title=Charakterystyka Produktu Leczniczego |url=https://rejestrymedyczne.ezdrowie.gov.pl/enwiki/api/rpl/medicinal-products/6729/characteristic |website=rejestrymedyczne.ezdrowie.gov.pl |access-date=14 May 2023 |archive-url=https://web.archive.org/web/20221020064552/https://rejestrymedyczne.ezdrowie.gov.pl/enwiki/api/rpl/medicinal-products/6729/characteristic |archive-date=October 20, 2022 |language=pl |url-status=live}}</ref> |
|||
| CASNo_Ref = {{cascite|correct|CAS}} |
|||
| CAS_number_Ref = {{cascite|correct|??}} |
|||
| CAS_number = 1420-55-9 |
|||
| ATC_prefix = R06 |
|||
| ATC_suffix = AD03 |
|||
| ATC_supplemental = |
|||
| PubChem = 5440 |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| DrugBank = DB00372 |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| ChemSpiderID = 5245 |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = 8ETK1WAF6R |
|||
| KEGG_Ref = {{keggcite|correct|kegg}} |
|||
| KEGG = D02354 |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
| ChEBI = 9544 |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
| ChEMBL = 1378 |
|||
=== Distribution === |
|||
<!--Chemical data--> |
|||
This drug is highly lipofilic and it binds with membranes and serum proteins (over 85%). It accumulates in organs with high blood flow and penetrates the placenta. It cannot be removed with dialysis. |
|||
| C=22 | H=29 | N=3 | S=2 |
|||
| molecular_weight = 399.618 g/mol |
|||
| smiles = S(c2cc1N(c3c(Sc1cc2)cccc3)CCCN4CCN(C)CC4)CC |
|||
| InChI = 1/C22H29N3S2/c1-3-26-18-9-10-22-20(17-18)25(19-7-4-5-8-21(19)27-22)12-6-11-24-15-13-23(2)14-16-24/h4-5,7-10,17H,3,6,11-16H2,1-2H3 |
|||
| InChIKey = XCTYLCDETUVOIP-UHFFFAOYAY |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChI = 1S/C22H29N3S2/c1-3-26-18-9-10-22-20(17-18)25(19-7-4-5-8-21(19)27-22)12-6-11-24-15-13-23(2)14-16-24/h4-5,7-10,17H,3,6,11-16H2,1-2H3 |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = XCTYLCDETUVOIP-UHFFFAOYSA-N |
|||
}} |
|||
=== Metabolism === |
|||
'''Thiethylperazine''' ('''Torecan''') is an [[antiemetic]] of the [[phenothiazine]] class. Though it was never licensed or used as an [[antipsychotic]], it may have such effects. |
|||
It is mainly metabolised in the liver and only 3% is eliminated unchanged. Torecan's [[half-life]] is 12 h. |
|||
== Teratogenicity == |
|||
Thiethylperazine activates the transport protein [[ABCC1]] that clears [[beta-amyloid]] from brains of mice. |
|||
In toxic doses above the terapeutic window, it increases the rate of cleft palate occurrence. |
|||
<ref> |
|||
{{cite web | |
|||
url=http://www.sciencedaily.com/releases/2011/09/110901134628.htm |
|||
|author=Science Daily |
|||
|title=Alzheimer Disease: Transport Protein ABCC1 Plays Key Role in Clearing Beta-Amyloid from Brains of Mice. | |
|||
date=September 1, 2011 | |
|||
accessdate=September 1, 2011 |
|||
}}</ref> |
|||
== Antipsychotic activity == |
|||
==References== |
|||
Theithylperazine may possess [[antipsychotic]] activity<ref>{{cite journal | vauthors = Rotrosen J, Angrist BM, Gershon S, Aronson M, Gruen P, Sachar EJ, Denning RK, Matthysse S, Stanley M, Wilk S | display-authors = 6 | title = Thiethylperazine; clinical antipsychotic efficacy and correlation with potency in predictive systems | journal = Archives of General Psychiatry | volume = 35 | issue = 9 | pages = 1112–1118 | date = September 1978 | pmid = 99115 | doi = 10.1001/archpsyc.1978.01770330086008 }}</ref> due to the antagonism of 5-HT<sub>2</sub> and D<sub>2</sub> receptors. It can cause [[extrapyramidal symptoms]].{{Citation needed|date=June 2023|reason=Slovak SPC for Torecan explicitly mentions the risk of extrapyramidal side effects.}} Nevertheless, it was never marketed as an antipsychotic. |
|||
One cause of acute [[dystonia]] occurred in a 19-year-old male patient after discontinuation of this drug.<ref>{{cite journal | vauthors = Khanderia U | title = Recurrent dystonic reactions induced by thiethylperazine | journal = Drug Intelligence & Clinical Pharmacy | volume = 19 | issue = 7–8 | pages = 550–551 | date = July 1985 | pmid = 4028959 | doi = 10.1177/106002808501900708 | s2cid = 44453678 }}</ref> |
|||
== Overdose == |
|||
Signs of acute thiethylperazine overdose include: extrapyramidal symptoms, confusion, [[convulsion]]s, [[respiratory depression]] and [[hypotension]]. |
|||
==Synthesis== |
|||
[[File:Thiethylperazine synthesis.svg|thumb|500px|center|[https://pharmaceutical-substances.thieme.com/ps/search-results?docUri=KD-20-0065 Thieme] Synthesis:<ref>{{cite journal | vauthors = Bourquin JP, Schwarb G, Gamboni G, Fischer R, Ruesch L, Guldimann S, Theus V, Schenker E, Renz J | display-authors = 6 | title = Synthesen auf dem Phenothiazin-Gebiet. 1. Mitteilung. Mercaptophenothiazin-Derivate. | journal = Helvetica Chimica Acta | date = 1958 | volume = 41 | issue = 4 | pages = 1061–1072 | doi = 10.1002/hlca.19580410419 }}</ref><ref>{{cite journal | vauthors = Bourquin JP, Schwarb G, Gamboni G, Fischer R, Ruesch L, Guldimann S, Theus V, Schenker E, Renz J | display-authors = 6 | title = Synthesen auf dem Phenothiazin-Gebiet. 2. Mitteilung. N-substituierte Mercaptophenothiazin-Derivate. | journal = Helvetica Chimica Acta | date = 1958 | volume = 41 | issue = 4 | pages = 1072–1108 | doi = 10.1002/hlca.19580410420 }}</ref> Patent:<ref>{{cite patent | country = US | number = 3336197 | gdate = 1967 | assign1 = Sandoz KK | inventor = Jany R, Bourquin jP, Gamboni G, Schwarb G | postscript = . }}</ref>]] |
|||
[[Goldberg reaction]] between 3-(ethylsulfanyl)aniline [1783-82-0] ('''1''') and [[2-chlorobenzoic acid]] [118-91-2] ('''2''') to give the diarylamine, [https://pubchem.ncbi.nlm.nih.gov/compound/82254530 CID:82254530] ('''3'''). The carboxyl in the anthranilic acid [[Residue (chemistry)|residue]], having performed its activating function, is then thermolytically removed to form [68083-49-8] ('''4'''). Upon treatment with sulfur and iodine, we get predominantly the [[phenothiazine]] [46815-10-5] ('''5'''); The rxn may well be aided by the presence of the electron donating [[thioether]] at the ''para''-position. Alkylation with 1-(ɣ̞-chloropropyl)-4-methylpiperazine [104-16-5] ('''6''') in the presence of sodamide affords ''Thiethylperazine'' ('''7'''). |
|||
== References == |
|||
{{reflist}} |
{{reflist}} |
||
{{Antiemetics}} |
{{Antiemetics}} |
||
{{Antipsychotics}} |
|||
{{Antihistamines}} |
{{Antihistamines}} |
||
{{Dopamine receptor modulators}} |
|||
{{Dopaminergics}} |
|||
{{Piperazines}} |
|||
{{Tricyclics}} |
{{Tricyclics}} |
||
[[Category: |
[[Category:Antiemetics]] |
||
[[Category:4-Methylpiperazin-1-yl compounds]] |
|||
[[Category:Phenothiazines]] |
[[Category:Phenothiazines]] |
||
[[Category:Thioethers]] |
[[Category:Thioethers]] |
||
{{gastrointestinal-drug-stub}} |
|||
{{respiratory-system-drug-stub}} |
{{respiratory-system-drug-stub}} |
||
{{nervous-system-drug-stub}} |
{{nervous-system-drug-stub}} |
||
Latest revision as of 12:45, 5 August 2024
 | |
| Clinical data | |
|---|---|
| Trade names | Torecan, Norzine |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 60% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.014.381 |
| Chemical and physical data | |
| Formula | C22H29N3S2 |
| Molar mass | 399.62 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Thiethylperazine (Torecan, Norzine) is an antiemetic[1] of the phenothiazine class. It is an antagonist of dopamine receptors (DRD1, DRD2, DRD4) as well as of 5-HT2A, 5-HT2C receptors, mAChRs (1 through 5), α1 adrenergic receptor and H1 receptor.
Thiethylperazine activates the transport protein ABCC1 that clears beta-amyloid from brains of mice.[2]
Pharmacokinetics
[edit]Distribution
[edit]This drug is highly lipofilic and it binds with membranes and serum proteins (over 85%). It accumulates in organs with high blood flow and penetrates the placenta. It cannot be removed with dialysis.
Metabolism
[edit]It is mainly metabolised in the liver and only 3% is eliminated unchanged. Torecan's half-life is 12 h.
Teratogenicity
[edit]In toxic doses above the terapeutic window, it increases the rate of cleft palate occurrence.
Antipsychotic activity
[edit]Theithylperazine may possess antipsychotic activity[4] due to the antagonism of 5-HT2 and D2 receptors. It can cause extrapyramidal symptoms.[citation needed] Nevertheless, it was never marketed as an antipsychotic.
One cause of acute dystonia occurred in a 19-year-old male patient after discontinuation of this drug.[5]
Overdose
[edit]Signs of acute thiethylperazine overdose include: extrapyramidal symptoms, confusion, convulsions, respiratory depression and hypotension.
Synthesis
[edit]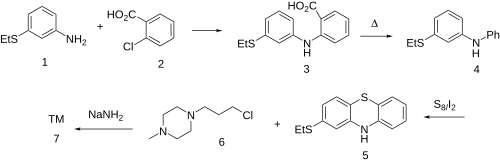
Goldberg reaction between 3-(ethylsulfanyl)aniline [1783-82-0] (1) and 2-chlorobenzoic acid [118-91-2] (2) to give the diarylamine, CID:82254530 (3). The carboxyl in the anthranilic acid residue, having performed its activating function, is then thermolytically removed to form [68083-49-8] (4). Upon treatment with sulfur and iodine, we get predominantly the phenothiazine [46815-10-5] (5); The rxn may well be aided by the presence of the electron donating thioether at the para-position. Alkylation with 1-(ɣ̞-chloropropyl)-4-methylpiperazine [104-16-5] (6) in the presence of sodamide affords Thiethylperazine (7).
References
[edit]- ^ Tamboline BL, Mcgillivray DC, Bogoch A (February 1965). "The Effects of Thiethylperazine Dimaleate (Torecan) on Nausea and Vomiting". Canadian Medical Association Journal. 92 (8): 422–423. PMC 1928133. PMID 14261157.
- ^ Krohn M, Lange C, Hofrichter J, Scheffler K, Stenzel J, Steffen J, et al. (October 2011). "Cerebral amyloid-β proteostasis is regulated by the membrane transport protein ABCC1 in mice". The Journal of Clinical Investigation. 121 (10): 3924–3931. doi:10.1172/JCI57867. PMC 3195473. PMID 21881209.
- "Alzheimer disease: Transport protein ABCC1 plays key role in clearing beta-amyloid from brains of mice". ScienceDaily (Press release). September 1, 2011.
- ^ "Charakterystyka Produktu Leczniczego". rejestrymedyczne.ezdrowie.gov.pl (in Polish). Archived from the original on October 20, 2022. Retrieved 14 May 2023.
- ^ Rotrosen J, Angrist BM, Gershon S, Aronson M, Gruen P, Sachar EJ, et al. (September 1978). "Thiethylperazine; clinical antipsychotic efficacy and correlation with potency in predictive systems". Archives of General Psychiatry. 35 (9): 1112–1118. doi:10.1001/archpsyc.1978.01770330086008. PMID 99115.
- ^ Khanderia U (July 1985). "Recurrent dystonic reactions induced by thiethylperazine". Drug Intelligence & Clinical Pharmacy. 19 (7–8): 550–551. doi:10.1177/106002808501900708. PMID 4028959. S2CID 44453678.
- ^ Bourquin JP, Schwarb G, Gamboni G, Fischer R, Ruesch L, Guldimann S, et al. (1958). "Synthesen auf dem Phenothiazin-Gebiet. 1. Mitteilung. Mercaptophenothiazin-Derivate". Helvetica Chimica Acta. 41 (4): 1061–1072. doi:10.1002/hlca.19580410419.
- ^ Bourquin JP, Schwarb G, Gamboni G, Fischer R, Ruesch L, Guldimann S, et al. (1958). "Synthesen auf dem Phenothiazin-Gebiet. 2. Mitteilung. N-substituierte Mercaptophenothiazin-Derivate". Helvetica Chimica Acta. 41 (4): 1072–1108. doi:10.1002/hlca.19580410420.
- ^ US 3336197, Jany R, Bourquin jP, Gamboni G, Schwarb G, issued 1967, assigned to Sandoz KK.
