Pyroxene: Difference between revisions
→See also: added "Rhodonite" and "Wollastonite" (both are pyroxenoid minerals) |
Replace template per TfD |
||
| (One intermediate revision by one other user not shown) | |||
| Line 4: | Line 4: | ||
The '''pyroxenes''' (commonly abbreviated '''Px''') are a group of important rock-forming [[Silicate minerals#Inosilicates|inosilicate]] [[mineral]]s found in many [[Igneous rock|igneous]] and [[metamorphic rock|metamorphic]] [[rock (geology)|rocks]]. Pyroxenes have the general formula {{chem2|XY(Si,Al)2O6}}, where X represents [[calcium]] (Ca), [[sodium]] (Na), [[iron]] (Fe(II)) or [[magnesium]] (Mg) and more rarely [[zinc]], [[manganese]] or [[lithium]], and Y represents ions of smaller size, such as [[chromium]] (Cr), [[aluminium]] (Al), [[magnesium]] (Mg), [[cobalt]] (Co), [[manganese]] (Mn), [[scandium]] (Sc), [[titanium]] (Ti), [[vanadium]] (V) or even iron (Fe(II) or Fe(III)). Although aluminium substitutes extensively for silicon in silicates such as [[feldspar]]s and [[amphibole]]s, the substitution occurs only to a limited extent in most pyroxenes. They share a common structure consisting of single chains of silica [[tetrahedra]]. Pyroxenes that crystallize in the [[monoclinic]] system are known as '''clinopyroxenes''' and those that crystallize in the [[orthorhombic]] system are known as '''orthopyroxenes'''. |
The '''pyroxenes''' (commonly abbreviated '''Px''') are a group of important rock-forming [[Silicate minerals#Inosilicates|inosilicate]] [[mineral]]s found in many [[Igneous rock|igneous]] and [[metamorphic rock|metamorphic]] [[rock (geology)|rocks]]. Pyroxenes have the general formula {{chem2|XY(Si,Al)2O6}}, where X represents [[calcium]] (Ca), [[sodium]] (Na), [[iron]] (Fe(II)) or [[magnesium]] (Mg) and more rarely [[zinc]], [[manganese]] or [[lithium]], and Y represents ions of smaller size, such as [[chromium]] (Cr), [[aluminium]] (Al), [[magnesium]] (Mg), [[cobalt]] (Co), [[manganese]] (Mn), [[scandium]] (Sc), [[titanium]] (Ti), [[vanadium]] (V) or even iron (Fe(II) or Fe(III)). Although aluminium substitutes extensively for silicon in silicates such as [[feldspar]]s and [[amphibole]]s, the substitution occurs only to a limited extent in most pyroxenes. They share a common structure consisting of single chains of silica [[tetrahedra]]. Pyroxenes that crystallize in the [[monoclinic]] system are known as '''clinopyroxenes''' and those that crystallize in the [[orthorhombic]] system are known as '''orthopyroxenes'''. |
||
The name ''pyroxene'' is derived from the [[Ancient Greek]] words for 'fire' ({{ |
The name ''pyroxene'' is derived from the [[Ancient Greek]] words for 'fire' ({{Wikt-lang|grc|πυρ}}, {{grc-transl|πυρ}}) and 'stranger' ({{Wikt-lang|grc|ξένος}}, {{grc-transl|ξένος}}). Pyroxenes were so named due to their presence in volcanic [[lava]]s, where they are sometimes found as [[phenocryst|crystals embedded]] in [[volcanic glass]]; it was assumed they were impurities in the glass, hence the name meaning "fire stranger". However, they are simply early-forming minerals that crystallized before the lava erupted. |
||
The [[upper mantle (Earth)|upper mantle]] of Earth is composed mainly of [[olivine]] and pyroxene minerals. Pyroxene and [[feldspar]] are the major minerals in [[basalt]], [[andesite]], and [[gabbro]] rocks.<ref>{{Cite journal|last1=Deegan|first1=Frances M.|last2=Whitehouse|first2=Martin J.|last3=Troll|first3=Valentin R.|last4=Budd|first4=David A.|last5=Harris|first5=Chris|last6=Geiger|first6=Harri|last7=Hålenius|first7=Ulf|date=2016-12-30|title=Pyroxene standards for SIMS oxygen isotope analysis and their application to Merapi volcano, Sunda arc, Indonesia|url=http://www.sciencedirect.com/science/article/pii/S0009254116305496|journal=Chemical Geology|language=en|volume=447|pages=1–10|doi=10.1016/j.chemgeo.2016.10.018|bibcode=2016ChGeo.447....1D|issn=0009-2541}}</ref><ref>{{Cite journal|last1=O’Driscoll|first1=Brian|last2=Stevenson|first2=Carl T. E.|last3=Troll|first3=Valentin R.|date=2008-05-15|title=Mineral Lamination Development in Layered Gabbros of the British Palaeogene Igneous Province: A Combined Anisotropy of Magnetic Susceptibility, Quantitative Textural and Mineral Chemistry Study|journal=Journal of Petrology|volume=49|issue=6|pages=1187–1221|doi=10.1093/petrology/egn022|issn=1460-2415|doi-access=free}}</ref> |
The [[upper mantle (Earth)|upper mantle]] of Earth is composed mainly of [[olivine]] and pyroxene minerals. Pyroxene and [[feldspar]] are the major minerals in [[basalt]], [[andesite]], and [[gabbro]] rocks.<ref>{{Cite journal|last1=Deegan|first1=Frances M.|last2=Whitehouse|first2=Martin J.|last3=Troll|first3=Valentin R.|last4=Budd|first4=David A.|last5=Harris|first5=Chris|last6=Geiger|first6=Harri|last7=Hålenius|first7=Ulf|date=2016-12-30|title=Pyroxene standards for SIMS oxygen isotope analysis and their application to Merapi volcano, Sunda arc, Indonesia|url=http://www.sciencedirect.com/science/article/pii/S0009254116305496|journal=Chemical Geology|language=en|volume=447|pages=1–10|doi=10.1016/j.chemgeo.2016.10.018|bibcode=2016ChGeo.447....1D|issn=0009-2541}}</ref><ref>{{Cite journal|last1=O’Driscoll|first1=Brian|last2=Stevenson|first2=Carl T. E.|last3=Troll|first3=Valentin R.|date=2008-05-15|title=Mineral Lamination Development in Layered Gabbros of the British Palaeogene Igneous Province: A Combined Anisotropy of Magnetic Susceptibility, Quantitative Textural and Mineral Chemistry Study|journal=Journal of Petrology|volume=49|issue=6|pages=1187–1221|doi=10.1093/petrology/egn022|issn=1460-2415|doi-access=free}}</ref> |
||
| Line 17: | Line 17: | ||
File:Diopside c scaled ibeam.jpg|Structure of pyroxene looking along the silica chains. "I-beams" are outlined in green. Silicon ions are oversized to emphasize the silicon chains. |
File:Diopside c scaled ibeam.jpg|Structure of pyroxene looking along the silica chains. "I-beams" are outlined in green. Silicon ions are oversized to emphasize the silicon chains. |
||
</gallery> |
</gallery> |
||
==Chemistry and nomenclature== |
==Chemistry and nomenclature== |
||
The chain silicate structure of the pyroxenes offers much flexibility in the incorporation of various [[cations]] and the names of the pyroxene minerals are primarily defined by their chemical composition. |
The chain silicate structure of the pyroxenes offers much flexibility in the incorporation of various [[cations]] and the names of the pyroxene minerals are primarily defined by their chemical composition. |
||
| Line 33: | Line 34: | ||
}} |
}} |
||
A typical pyroxene has mostly silicon in the tetrahedral site and predominately ions with a charge of +2 in both the X and Y sites, giving the approximate formula {{chem2|XYT2O6}}. The names of the common calcium{{ndash}}iron{{ndash}}magnesium pyroxenes are defined in the 'pyroxene quadrilateral'. The [[enstatite|enstatite-ferrosilite]] series ({{chem2|[Mg,Fe]SiO3}}) includes the common rock-forming mineral [[ |
A typical pyroxene has mostly silicon in the tetrahedral site and predominately ions with a charge of +2 in both the X and Y sites, giving the approximate formula {{chem2|XYT2O6}}. The names of the common calcium{{ndash}}iron{{ndash}}magnesium pyroxenes are defined in the 'pyroxene quadrilateral'. The [[enstatite|enstatite-ferrosilite]] series ({{chem2|[Mg,Fe]SiO3}}) includes the common rock-forming mineral [[hypersthene]], contains up to 5 mol.% calcium and exists in three polymorphs, [[orthorhombic]] orthoenstatite and protoenstatite and [[monoclinic]] clinoenstatite (and the ferrosilite equivalents). Increasing the calcium content prevents the formation of the orthorhombic phases and [[pigeonite]] ({{chem2|[Mg,Fe,Ca][Mg,Fe]Si2O6}}) only crystallises in the monoclinic system. There is not complete solid solution in calcium content and Mg-Fe-Ca pyroxenes with calcium contents between about 15 and 25 mol.% are not stable with respect to a pair of exolved crystals. This leads to a [[miscibility gap]] between pigeonite and [[augite]] compositions. There is an arbitrary separation between augite and the [[diopside|diopside-hedenbergite]] ({{chem2|CaMgSi2O6{{snd}}CaFeSi2O6}}) solid solution. The divide is taken at >45 mol.% Ca. As the calcium ion cannot occupy the Y site, pyroxenes with more than 50 mol.% calcium are not possible. A related mineral [[wollastonite]] has the formula of the hypothetical calcium end member ({{chem2|Ca2Si2O6}}) but important structural differences mean that it is instead classified as a pyroxenoid. |
||
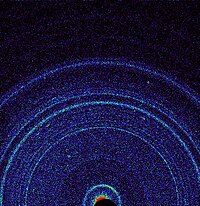
Magnesium, calcium and iron are by no means the only cations that can occupy the X and Y sites in the pyroxene structure. A second important series of pyroxene minerals are the sodium-rich pyroxenes, corresponding to the 'pyroxene triangle' nomenclature. The inclusion of sodium, which has a charge of +1, into the pyroxene implies the need for a mechanism to make up the "missing" positive charge. In [[jadeite]] and [[aegirine]] this is added by the inclusion of a +3 cation (aluminium and iron(III) respectively) on the Y site. Sodium pyroxenes with more than 20 mol.% calcium, magnesium or iron(II) components are known as [[omphacite]] and [[aegirine-augite]]. With 80% or more of these components the pyroxene is classified using the quadrilateral diagram.[[File:PIA16217-MarsCuriosityRover-1stXRayView-20121017.jpg|thumb|200px|right|First [[X-ray crystallography#Mineralogy and metallurgy|X-ray diffraction view]] of [[Martian soil]] – [[CheMin|CheMin analysis]] reveals [[feldspar]], pyroxenes, [[olivine]] and more ([[Curiosity rover]] at "[[Rocknest (Mars)|Rocknest]]")<ref name="NASA-20121030">{{cite web |last=Brown |first=Dwayne |title=NASA Rover's First Soil Studies Help Fingerprint Martian Minerals |url=http://www.nasa.gov/home/hqnews/2012/oct/HQ_12-383_Curiosity_CheMin.html |date=October 30, 2012 |publisher=[[NASA]] |access-date=October 31, 2012}}</ref>]] |
Magnesium, calcium and iron are by no means the only cations that can occupy the X and Y sites in the pyroxene structure. A second important series of pyroxene minerals are the sodium-rich pyroxenes, corresponding to the 'pyroxene triangle' nomenclature. The inclusion of sodium, which has a charge of +1, into the pyroxene implies the need for a mechanism to make up the "missing" positive charge. In [[jadeite]] and [[aegirine]] this is added by the inclusion of a +3 cation (aluminium and iron(III) respectively) on the Y site. Sodium pyroxenes with more than 20 mol.% calcium, magnesium or iron(II) components are known as [[omphacite]] and [[aegirine-augite]]. With 80% or more of these components the pyroxene is classified using the quadrilateral diagram.[[File:PIA16217-MarsCuriosityRover-1stXRayView-20121017.jpg|thumb|200px|right|First [[X-ray crystallography#Mineralogy and metallurgy|X-ray diffraction view]] of [[Martian soil]] – [[CheMin|CheMin analysis]] reveals [[feldspar]], pyroxenes, [[olivine]] and more ([[Curiosity rover]] at "[[Rocknest (Mars)|Rocknest]]")<ref name="NASA-20121030">{{cite web |last=Brown |first=Dwayne |title=NASA Rover's First Soil Studies Help Fingerprint Martian Minerals |url=http://www.nasa.gov/home/hqnews/2012/oct/HQ_12-383_Curiosity_CheMin.html |date=October 30, 2012 |publisher=[[NASA]] |access-date=October 31, 2012}}</ref>]] |
||
Latest revision as of 22:03, 13 August 2024

The pyroxenes (commonly abbreviated Px) are a group of important rock-forming inosilicate minerals found in many igneous and metamorphic rocks. Pyroxenes have the general formula XY(Si,Al)2O6, where X represents calcium (Ca), sodium (Na), iron (Fe(II)) or magnesium (Mg) and more rarely zinc, manganese or lithium, and Y represents ions of smaller size, such as chromium (Cr), aluminium (Al), magnesium (Mg), cobalt (Co), manganese (Mn), scandium (Sc), titanium (Ti), vanadium (V) or even iron (Fe(II) or Fe(III)). Although aluminium substitutes extensively for silicon in silicates such as feldspars and amphiboles, the substitution occurs only to a limited extent in most pyroxenes. They share a common structure consisting of single chains of silica tetrahedra. Pyroxenes that crystallize in the monoclinic system are known as clinopyroxenes and those that crystallize in the orthorhombic system are known as orthopyroxenes.
The name pyroxene is derived from the Ancient Greek words for 'fire' (πυρ, pur) and 'stranger' (ξένος, xénos). Pyroxenes were so named due to their presence in volcanic lavas, where they are sometimes found as crystals embedded in volcanic glass; it was assumed they were impurities in the glass, hence the name meaning "fire stranger". However, they are simply early-forming minerals that crystallized before the lava erupted.
The upper mantle of Earth is composed mainly of olivine and pyroxene minerals. Pyroxene and feldspar are the major minerals in basalt, andesite, and gabbro rocks.[1][2]
Structure
[edit]Pyroxenes are the most common single-chain silicate minerals. (The only other important group of single-chain silicates, the pyroxenoids, are much less common.) Their structure consists of parallel chains of negatively-charged silica tetrahedra bonded together by metal cations. In other words, each silicon ion in a pyroxene crystal is surrounded by four oxygen ions forming a tetrahedron around the relatively small silicon ion. Each silicon ion shares two oxygen ions with neighboring silicon ions in the chain.[3]
The tetrahedra in the chain all face in the same direction, so that two oxygen ions are located on one face of the chain for every oxygen ion on the other face of the chain. The oxygen ions on the narrower face are described as apical oxygen ions. Pairs of chains are bound together on their apical sides by Y cations, with each Y cation surrounded by six oxygen ions. The resulting pairs of single chains have sometimes been likened to I-beams. The I-beams interlock, with additional X cations bonding the outer faces of the I-beams to neighboring I-beams and providing the remaining charge balance. This binding is relatively weak and gives pyroxenes their characteristic cleavage.[3]
-
A single chain of silicon tetrahedra viewed in the [100] direction
-
A single chain of silica tetrahedra viewed in the [010] direction
-
Structure of pyroxene looking along the silica chains. "I-beams" are outlined in green. Silicon ions are oversized to emphasize the silicon chains.
Chemistry and nomenclature
[edit]The chain silicate structure of the pyroxenes offers much flexibility in the incorporation of various cations and the names of the pyroxene minerals are primarily defined by their chemical composition. Pyroxene minerals are named according to the chemical species occupying the X (or M2) site, the Y (or M1) site, and the tetrahedral T site. Cations in Y (M1) site are closely bound to 6 oxygens in octahedral coordination. Cations in the X (M2) site can be coordinated with 6 to 8 oxygen atoms, depending on the cation size. Twenty mineral names are recognised by the International Mineralogical Association's Commission on New Minerals and Mineral Names and 105 previously used names have been discarded.[4]
A typical pyroxene has mostly silicon in the tetrahedral site and predominately ions with a charge of +2 in both the X and Y sites, giving the approximate formula XYT2O6. The names of the common calcium–iron–magnesium pyroxenes are defined in the 'pyroxene quadrilateral'. The enstatite-ferrosilite series ([Mg,Fe]SiO3) includes the common rock-forming mineral hypersthene, contains up to 5 mol.% calcium and exists in three polymorphs, orthorhombic orthoenstatite and protoenstatite and monoclinic clinoenstatite (and the ferrosilite equivalents). Increasing the calcium content prevents the formation of the orthorhombic phases and pigeonite ([Mg,Fe,Ca][Mg,Fe]Si2O6) only crystallises in the monoclinic system. There is not complete solid solution in calcium content and Mg-Fe-Ca pyroxenes with calcium contents between about 15 and 25 mol.% are not stable with respect to a pair of exolved crystals. This leads to a miscibility gap between pigeonite and augite compositions. There is an arbitrary separation between augite and the diopside-hedenbergite (CaMgSi2O6 − CaFeSi2O6) solid solution. The divide is taken at >45 mol.% Ca. As the calcium ion cannot occupy the Y site, pyroxenes with more than 50 mol.% calcium are not possible. A related mineral wollastonite has the formula of the hypothetical calcium end member (Ca2Si2O6) but important structural differences mean that it is instead classified as a pyroxenoid.
Magnesium, calcium and iron are by no means the only cations that can occupy the X and Y sites in the pyroxene structure. A second important series of pyroxene minerals are the sodium-rich pyroxenes, corresponding to the 'pyroxene triangle' nomenclature. The inclusion of sodium, which has a charge of +1, into the pyroxene implies the need for a mechanism to make up the "missing" positive charge. In jadeite and aegirine this is added by the inclusion of a +3 cation (aluminium and iron(III) respectively) on the Y site. Sodium pyroxenes with more than 20 mol.% calcium, magnesium or iron(II) components are known as omphacite and aegirine-augite. With 80% or more of these components the pyroxene is classified using the quadrilateral diagram.
A wide range of other cations that can be accommodated in the different sites of pyroxene structures.
| T | Si | Al | Fe3+ | ||||||||||||||
| Y | Al | Fe3+ | Ti4+ | Cr | V | Ti3+ | Zr | Sc | Zn | Mg | Fe2+ | Mn | |||||
| X | Mg | Fe2+ | Mn | Li | Ca | Na |
In assigning ions to sites, the basic rule is to work from left to right in this table, first assigning all silicon to the T site and then filling the site with the remaining aluminium and finally iron(III); extra aluminium or iron can be accommodated in the Y site and bulkier ions on the X site.
Not all the resulting mechanisms to achieve charge neutrality follow the sodium example above, and there are several alternative schemes:
- Coupled substitutions of 1+ and 3+ ions on the X and Y sites respectively. For example, Na and Al give the jadeite (NaAlSi2O6) composition.
- Coupled substitution of a 1+ ion on the X site and a mixture of equal numbers of 2+ and 4+ ions on the Y site. This leads to e.g., NaFe2+0.5Ti4+0.5Si2O6.
- The Tschermak substitution where a 3+ ion occupies the Y site and a T site leading to e.g., CaAlAlSiO6.
In nature, more than one substitution may be found in the same mineral.
Pyroxene minerals
[edit]


- Clinopyroxenes (monoclinic)
- Aegirine, NaFe3+Si2O6
- Augite, (Ca,Na)(Mg,Fe,Al,Ti)(Si,Al)2O6
- Clinoenstatite, MgSiO3
- Diopside, CaMgSi2O6
- Esseneite, CaFe3+[AlSiO6]
- Hedenbergite, CaFe2+Si2O6
- Jadeite, Na(Al,Fe3+)Si2O6
- Jervisite, (Na,Ca,Fe2+)(Sc,Mg,Fe2+)Si2O6
- Johannsenite, CaMn2+Si2O6
- Kanoite, Mn2+(Mg,Mn2+)Si2O6
- Kosmochlor, NaCrSi2O6
- Namansilite, NaMn3+Si2O6
- Natalyite, NaV3+Si2O6
- Omphacite, (Ca,Na)(Mg,Fe2+,Al)Si2O6
- Petedunnite, Ca(Zn,Mn2+,Mg,Fe2+)Si2O6
- Pigeonite, (Ca,Mg,Fe)(Mg,Fe)Si2O6
- Spodumene, LiAl(SiO3)2
- Orthopyroxenes (orthorhombic)
- Enstatite, Mg2Si2O6
- Bronzite, intermediate between enstatite and hypersthene
- Hypersthene, (Mg,Fe)SiO3
- Eulite, intermediate between hypersthene and ferrosilite
- Ferrosilite, Fe2Si2O6
- Donpeacorite, (MgMn)MgSi2O6
- Nchwaningite, Mn2+2SiO3(OH)2·(H2O)
See also
[edit]- Clinopyroxene thermobarometry
- Emerald – sometimes substituted in jewelry by hiddenite, a green variety of spodumene.
- Rhodonite
- Wollastonite
References
[edit]- ^ Deegan, Frances M.; Whitehouse, Martin J.; Troll, Valentin R.; Budd, David A.; Harris, Chris; Geiger, Harri; Hålenius, Ulf (2016-12-30). "Pyroxene standards for SIMS oxygen isotope analysis and their application to Merapi volcano, Sunda arc, Indonesia". Chemical Geology. 447: 1–10. Bibcode:2016ChGeo.447....1D. doi:10.1016/j.chemgeo.2016.10.018. ISSN 0009-2541.
- ^ O’Driscoll, Brian; Stevenson, Carl T. E.; Troll, Valentin R. (2008-05-15). "Mineral Lamination Development in Layered Gabbros of the British Palaeogene Igneous Province: A Combined Anisotropy of Magnetic Susceptibility, Quantitative Textural and Mineral Chemistry Study". Journal of Petrology. 49 (6): 1187–1221. doi:10.1093/petrology/egn022. ISSN 1460-2415.
- ^ a b Nesse, William D. (2000). Introduction to mineralogy. New York: Oxford University Press. p. 261. ISBN 9780195106916.
- ^ Morimoto, N.; Fabries, J.; Ferguson, A.K.; Ginzburg, I.V.; Ross, M.; Seifeit, F.A.; Zussman, J. (1989). "Nomenclature of pyroxenes" (PDF). Canadian Mineralogist. 27: 143–156. Archived from the original (PDF) on 9 March 2008.
- ^ Brown, Dwayne (October 30, 2012). "NASA Rover's First Soil Studies Help Fingerprint Martian Minerals". NASA. Retrieved October 31, 2012.
- C. Michael Hogan (2010). Calcium. eds. A. Jorgensen, C. Cleveland. Encyclopedia of Earth. National Council for Science and the Environment.
External links
[edit]- Mineral Galleries
- Video Section: Lunar Explorers (link to youtube: The Lunar Crust)
- . Encyclopædia Britannica. Vol. 22 (11th ed.). 1911. p. 696.

![A single chain of silica tetrahedra viewed in the [010] direction](/upwiki/wikipedia/commons/thumb/d/db/Ino_b.jpg/120px-Ino_b.jpg)



