Bis(triethoxysilylpropyl)tetrasulfide: Difference between revisions
No edit summary Tags: Mobile edit Mobile web edit |
Calle Cool (talk | contribs) →Synthesis and reactivity: reaction |
||
| (18 intermediate revisions by 12 users not shown) | |||
| Line 1: | Line 1: | ||
{{Chembox |
{{Chembox |
||
| ImageFile = Si69.svg |
| ImageFile = Si69.svg |
||
| Name=Bis(triethoxysilylpropyl){{shy}}tetrasulfide |
|||
| ImageSize = |
| ImageSize = |
||
| ImageAlt = |
| ImageAlt = |
||
| PIN = [Tetrasulfanediyldi(propane-3,1-diyl)]bis(triethoxysilane) |
|||
| IUPACName = |
|||
| OtherNames = |
| OtherNames = {{ubl|Bis[3-(triethoxysilyl)propyl]tetrasulfide|Bis(3-triethoxysilylpropyl)tetrasulfane|Si-69|TESPT}} |
||
|Section1={{Chembox Identifiers |
|Section1={{Chembox Identifiers |
||
| CASNo = 40372-72-3 |
| CASNo = 40372-72-3 |
||
| PubChem = |
| PubChem = 162012 |
||
| |
| EC_number = 254-896-5 |
||
| UNII = J98V193ZRY |
|||
| ChEMBL = 3188179 |
|||
| ChemSpiderID = 142291 |
|||
| StdInChI = 1S/C18H42O6S4Si2/c1-7-19-29(20-8-2,21-9-3)17-13-15-25-27-28-26-16-14-18-30(22-10-4,23-11-5)24-12-6/h7-18H2,1-6H3 |
|||
| StdInChIKey = VTHOKNTVYKTUPI-UHFFFAOYSA-N |
|||
| SMILES = CCO[Si](CCCSSSSCCC[Si](OCC)(OCC)OCC)(OCC)OCC |
|||
}} |
|||
|Section2={{Chembox Properties |
|Section2={{Chembox Properties |
||
| Formula = C<sub>18</sub>H<sub>42</sub>O<sub>6</sub>S<sub>4</sub>Si<sub>2</sub> |
| Formula = C<sub>18</sub>H<sub>42</sub>O<sub>6</sub>S<sub>4</sub>Si<sub>2</sub> |
||
| MolarMass = 538.95 |
| MolarMass = 538.95 |
||
| Appearance = |
| Appearance =yellow syrup |
||
| Density = 1.08 g/cm<sup>3</sup> |
| Density = 1.08 g/cm<sup>3</sup> |
||
| MeltingPt = |
| MeltingPt = |
||
| Line 23: | Line 31: | ||
}} |
}} |
||
'''Bis(triethoxysilylpropyl)tetrasulfide''' is an [[organosulfur compound]] with the formula S<sub>4</sub>[C<sub>3</sub>H<sub>6</sub>Si(OEt)<sub>3</sub>]<sub>2</sub> ([[Ethyl group|Et]] = C<sub>2</sub>H<sub>5</sub>). The molecule consists of two trialkoxysilyl propyl groups linked with a [[polysulfide]]. It is often sold as a mixture with the trisulfide. The compound is a colorless viscous liquid that is soluble in ordinary organic solvents such as toluene. Commercial samples often are yellowish. The compound is added to rubber compositions that contain [[silica]] filler.<ref>Kohjiya |
'''Bis(triethoxysilylpropyl)tetrasulfide''' is an [[organosulfur compound]] with the formula S<sub>4</sub>[C<sub>3</sub>H<sub>6</sub>Si(OEt)<sub>3</sub>]<sub>2</sub> ([[Ethyl group|Et]] = C<sub>2</sub>H<sub>5</sub>). The molecule consists of two trialkoxysilyl propyl groups linked with a [[polysulfide]]. It is often sold as a mixture with the trisulfide. The compound is a colorless viscous liquid that is soluble in ordinary organic solvents such as [[toluene]]. Commercial samples often are yellowish. The compound is added to rubber compositions that contain [[silica]] filler.<ref>{{cite journal | last1 = Kohjiya | first1 = Shinzo | last2 = Ikeda | first2 = Yuko | year = 2000 | title = Reinforcement of general-purpose grade rubbers by silica generated in situ | journal = Rubber Chemistry and Technology | volume = 73 | issue = 3 | pages = 534–550 | doi=10.5254/1.3547604}}</ref><ref>{{cite journal | last1 = Wolff | first1 = Siegfried | year = 1996 | title = Chemical aspects of rubber reinforcement by fillers | journal = Rubber Chemistry and Technology | volume = 69 | issue = 3 | pages = 325–346 | doi=10.5254/1.3538376}}</ref><ref>{{cite journal|author=Vilmin, F.|author2= Bottero, I.|author3= Travert, A.|author4= Malicki, N.|author5= Gaboriaud, F.|author6= Trivella, A.|author7= Thibault-Starzyk, F.|title=Reactivity of Bis[3-(triethoxysilyl)propyl] Tetrasulfide (TESPT) Silane Coupling Agent over Hydrated Silica: Operando IR Spectroscopy and Chemometrics Study|journal=The Journal of Physical Chemistry C|year=2014|volume=118|issue= 8|page=4056–4071|doi=10.1021/jp408600h}}</ref> |
||
==Synthesis and reactivity== |
==Synthesis and reactivity== |
||
[[file:Synthesis Bis(triethoxysilylpropyl)tetrasulfide.svg|thumb|left|Reaction]] |
|||
The compound was first prepared by the reaction of |
The compound was first prepared by the reaction of 3-(triethoxysilyl)propyl chloride with [[sodium tetrasulfide]]:<ref>Thurn, Friedrich; Meyer-Simon, Eugen; Michel, Rudolf "Verfahren zur Herstellung von Organosiliziumverbindungen (Continuous manufacture of bis[3-(triethoxysilyl)propyl] tetrasulfide)" Ger. Offen. (1973), DE 2212239 A1 19731004.</ref> |
||
:Na<sub>2</sub>S<sub>4</sub> + 2 ClC<sub>3</sub>H<sub>6</sub>Si(OEt)<sub>3</sub> → S<sub>4</sub>[C<sub>3</sub>H<sub>6</sub>Si(OEt)<sub>3</sub>]<sub>2</sub> + 2 NaCl |
:Na<sub>2</sub>S<sub>4</sub> + 2 ClC<sub>3</sub>H<sub>6</sub>Si(OEt)<sub>3</sub> → S<sub>4</sub>[C<sub>3</sub>H<sub>6</sub>Si(OEt)<sub>3</sub>]<sub>2</sub> + 2 NaCl |
||
Bis(triethoxysilylpropyl)tetrasulfide is a bifunctional molecule in that it contains two kinds of reactive [[functional group]]s. The tetrasulfide group is a [[polysulfide]], which means that it consists of a chain of sulfur atoms. S-S bonds are susceptible to reduction (to thiols), attachment to metals (e.g., for protection against [[corrosion]]), and [[vulcanization]]. The triethoxysilyl groups are susceptible to [[hydrolysis]], resulting in [[cross-link]]ing via [[sol-gel#polymerization|sol-gel condensation]]. In the usual application of |
Bis(triethoxysilylpropyl)tetrasulfide is a bifunctional molecule in that it contains two kinds of reactive [[functional group]]s. The tetrasulfide group is a [[polysulfide]], which means that it consists of a chain of sulfur atoms. S-S bonds are susceptible to reduction (to thiols), attachment to metals (e.g., for protection against [[corrosion]]), and [[vulcanization]]. The triethoxysilyl groups are susceptible to [[hydrolysis]], resulting in [[cross-link]]ing via [[sol-gel#polymerization|sol-gel condensation]]. In the usual application of this chemical, the hydrolyzed siloxy groups attach to silica particles and the polysulfide groups link to the organic polymer.<ref>{{cite journal|author=Choi, S.-S.|author2= Kim, I.-S.|author3= Woo, C.-S.|title=Influence of TESPT Content on Crosslink Types and Rheological Behaviors of Natural rubber compounds reinforced with Silica|journal=Journal of Applied Polymer Science|year=2007|volume=106|issue= 4|page=2753–2758|doi=10.1002/app.25744}}</ref> |
||
==References== |
==References== |
||
{{Reflist}} |
|||
<references /> |
|||
[[Category:Sulfur compounds]] |
[[Category:Sulfur compounds]] |
||
[[Category:Corrosion]] |
[[Category:Corrosion inhibitors]] |
||
[[Category:Ethoxides]] |
[[Category:Ethoxides]] |
||
[[Category: |
[[Category:Organosilicon compounds]] |
||
[[Category:Orthoesters]] |
|||
Latest revision as of 15:01, 20 August 2024

| |
| Names | |
|---|---|
| Preferred IUPAC name
[Tetrasulfanediyldi(propane-3,1-diyl)]bis(triethoxysilane) | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.049.888 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H42O6S4Si2 | |
| Molar mass | 538.95 |
| Appearance | yellow syrup |
| Density | 1.08 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bis(triethoxysilylpropyl)tetrasulfide is an organosulfur compound with the formula S4[C3H6Si(OEt)3]2 (Et = C2H5). The molecule consists of two trialkoxysilyl propyl groups linked with a polysulfide. It is often sold as a mixture with the trisulfide. The compound is a colorless viscous liquid that is soluble in ordinary organic solvents such as toluene. Commercial samples often are yellowish. The compound is added to rubber compositions that contain silica filler.[1][2][3]
Synthesis and reactivity
[edit]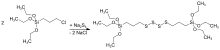
The compound was first prepared by the reaction of 3-(triethoxysilyl)propyl chloride with sodium tetrasulfide:[4]
- Na2S4 + 2 ClC3H6Si(OEt)3 → S4[C3H6Si(OEt)3]2 + 2 NaCl
Bis(triethoxysilylpropyl)tetrasulfide is a bifunctional molecule in that it contains two kinds of reactive functional groups. The tetrasulfide group is a polysulfide, which means that it consists of a chain of sulfur atoms. S-S bonds are susceptible to reduction (to thiols), attachment to metals (e.g., for protection against corrosion), and vulcanization. The triethoxysilyl groups are susceptible to hydrolysis, resulting in cross-linking via sol-gel condensation. In the usual application of this chemical, the hydrolyzed siloxy groups attach to silica particles and the polysulfide groups link to the organic polymer.[5]
References
[edit]- ^ Kohjiya, Shinzo; Ikeda, Yuko (2000). "Reinforcement of general-purpose grade rubbers by silica generated in situ". Rubber Chemistry and Technology. 73 (3): 534–550. doi:10.5254/1.3547604.
- ^ Wolff, Siegfried (1996). "Chemical aspects of rubber reinforcement by fillers". Rubber Chemistry and Technology. 69 (3): 325–346. doi:10.5254/1.3538376.
- ^ Vilmin, F.; Bottero, I.; Travert, A.; Malicki, N.; Gaboriaud, F.; Trivella, A.; Thibault-Starzyk, F. (2014). "Reactivity of Bis[3-(triethoxysilyl)propyl] Tetrasulfide (TESPT) Silane Coupling Agent over Hydrated Silica: Operando IR Spectroscopy and Chemometrics Study". The Journal of Physical Chemistry C. 118 (8): 4056–4071. doi:10.1021/jp408600h.
- ^ Thurn, Friedrich; Meyer-Simon, Eugen; Michel, Rudolf "Verfahren zur Herstellung von Organosiliziumverbindungen (Continuous manufacture of bis[3-(triethoxysilyl)propyl] tetrasulfide)" Ger. Offen. (1973), DE 2212239 A1 19731004.
- ^ Choi, S.-S.; Kim, I.-S.; Woo, C.-S. (2007). "Influence of TESPT Content on Crosslink Types and Rheological Behaviors of Natural rubber compounds reinforced with Silica". Journal of Applied Polymer Science. 106 (4): 2753–2758. doi:10.1002/app.25744.
