Ecdysone: Difference between revisions
Polinizador (talk | contribs) mNo edit summary |
Citation bot (talk | contribs) Added pmc. | Use this bot. Report bugs. | Suggested by Headbomb | #UCB_toolbar |
||
| (23 intermediate revisions by 13 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Precursor of an insect hormone}} |
|||
{{chembox |
|||
{{Chembox |
|||
| Watchedfields = changed |
| Watchedfields = changed |
||
| verifiedrevid = 461092187 |
| verifiedrevid = 461092187 |
||
| Line 8: | Line 9: | ||
| ImageSize1 = 240 |
| ImageSize1 = 240 |
||
| ImageAlt1 = Ball-and-stick model of the ecdysone molecule |
| ImageAlt1 = Ball-and-stick model of the ecdysone molecule |
||
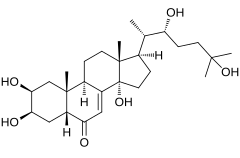
| IUPACName = (22''R'')-2β,3β,14α,22,25- |
| IUPACName = (22''R'')-2β,3β,14α,22,25-Pentahydroxy-5β-cholest-7-en-6-one |
||
| SystematicName = ( |
| SystematicName = (1''R'',3a''S'',5a''R'',7''R'',8''S'',9a''R'',9b''R'',11a''R'')-1-[(2''S'',3''R'')-3,6-Dihydroxy-6-methylheptan-2-yl]-3a,7,8-trihydroxy-9a,11a-dimethyl-1,2,3,3a,5a,6,7,8,9,9a,9b,10,11,11a-tetradecahydro-5''H''-cyclopenta[''a'']phenanthren-5-one |
||
| OtherNames = |
| OtherNames = |
||
| Section1 = {{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| Line 22: | Line 23: | ||
| CASNo_Ref = {{cascite|correct|CAS}} |
| CASNo_Ref = {{cascite|correct|CAS}} |
||
| CASNo = 3604-87-3 |
| CASNo = 3604-87-3 |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = RH692X7B7B |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
||
| ChEMBL = 549300 |
| ChEMBL = 549300 |
||
| Line 42: | Line 45: | ||
| AutoignitionPt = }} |
| AutoignitionPt = }} |
||
}} |
}} |
||
'''Ecdysone''' is a |
'''Ecdysone''' is a{{clarify|reason=the?|date=October 2022}} [[prohormone]] of the major insect [[ecdysis|molting]] hormone [[20-hydroxyecdysone]], secreted from the [[prothoracic gland]]s. It is of [[steroid]]al structure. Insect molting hormones (ecdysone and its homologues) are generally called [[ecdysteroid]]s. Ecdysteroids act as moulting hormones of [[arthropod]]s but also occur in other related [[Phylum|phyla]] where they can play different roles.<ref name="Hoffmeister">{{cite journal | id=Physiologisch-Chemisches Institut der Universität Marburg/Lahn | volume=20 | pages=130–133 | first3=Helmut | last3=Ammon | first2=Clemens | first1=Hans | last2=Rufer | last1=Hoffmeister | journal=Zeitschrift für Naturforschung B | date=February 1, 1965 | pmid=14345159 | doi=10.1515/znb-1965-0207 | title=Ausscheidung von Ecdyson bei Insekten | issue=2 | s2cid=95079385 | trans-title=Excretion of Ecdysone by Insects| doi-access=free }}</ref><ref>Karlson, P; Hoffmeister, H (March, 1963). On the biogenesis of ecdyson. I. Conversion of cholesterol into ecdyson. ''Hoppe Seylers Z Physiol Chem.'', 331, 289–300. In German. PMID 1396254</ref> In ''[[Drosophila melanogaster]]'', an increase in ecdysone concentration induces the expression of genes coding for proteins that the larva requires. It causes chromosome puffs (sites of high expression) to form in [[polytene chromosomes]]. Recent findings in the laboratory of [[Chris Q. Doe]] have found a novel role of this [[hormone]] in regulating temporal [[gene transitions]] within [[neural stem cells]] of the fruit fly.<ref>{{cite journal |doi=10.7554/eLife.26287 |pmid=28394252 |pmc=5403213 |title=Steroid hormone induction of temporal gene expression in Drosophila brain neuroblasts generates neuronal and glial diversity |journal=eLife |volume=6 |year=2017 |last1=Syed |first1=Mubarak Hussain |last2=Mark |first2=Brandon |last3=Doe |first3=Chris Q. |doi-access=free }}</ref> |
||
Ecdysone and other ecdysteroids also appear in many plants mostly as a protection agent ([[toxins]] or [[antifeedant]]s) against [[herbivorous]] insects.<ref>{{ cite journal | vauthors = Dinan L, Savchenko T, Whiting P | title = On the distribution of phytoecdysteroids in plants | journal = Cellular and Molecular Life Sciences | year = 2001 | volume = 58 | issue = 8 | pages = 1121–1132 | doi = 10.1007/PL00000926 | pmid = 11529504 }}</ref> These [[phytoecdysteroid]]s have been reputed to have [[medicinal]] value |
Ecdysone and other ecdysteroids also appear in many plants mostly as a protection agent ([[toxins]] or [[antifeedant]]s) against [[herbivorous]] insects.<ref>{{ cite journal | vauthors = Dinan L, Savchenko T, Whiting P | title = On the distribution of phytoecdysteroids in plants | journal = Cellular and Molecular Life Sciences | year = 2001 | volume = 58 | issue = 8 | pages = 1121–1132 | doi = 10.1007/PL00000926 | pmid = 11529504 | pmc = 11337386 | s2cid = 8496934 }}</ref> These [[phytoecdysteroid]]s have been reputed to have [[medicinal]] value. They are part of herbal [[adaptogen]]ic remedies like [[Cordyceps]], yet an ecdysteroid precursor in plants has been shown to have [[cytotoxic]] properties<ref>Wang YS, Yang JH, Luo SD, Zhang HB, Li L, Molecules. 2007;12(3):536-42</ref> as well as [[antioxidant]] properties on [[lipid peroxidation]].<ref>{{cite journal | vauthors = Kuzmenko AI, Niki E, Noguchi N | title = New functions of 20-hydroxyecdysone in lipid peroxidation | journal = Journal of Oleo Science | volume = 50 | issue = 6 | pages = 497–506 | year = 2001 | doi = 10.5650/jos.50.497 | url = https://www.jstage.jst.go.jp/article/jos/50/6/50_6_497/_pdf | doi-access = free }}</ref> |
||
[[Tebufenozide]], sold under the [[Bayer]] trademark MIMIC,<ref>[https://apvma.gov.au/node/14051 apvma.gov.au: "Tebufenozide in the product Mimic 700 WP Insecticide, Mimic 240 SC Insecticide"]</ref> has ecdysteroid activity although its chemical structure has little resemblance to the ecdysteroids.{{ |
[[Tebufenozide]], sold under the [[Bayer]] trademark MIMIC,<ref>[https://apvma.gov.au/node/14051 apvma.gov.au: "Tebufenozide in the product Mimic 700 WP Insecticide, Mimic 240 SC Insecticide"]</ref> has ecdysteroid activity although its chemical structure has little resemblance to the ecdysteroids.<ref>{{Cite book | doi = 10.1021/bk-2000-0767.ch002 | chapter = Tebufenozide: A Novel Caterpillar Control Agent with Unusually High Target Selectivity | title = Green Chemical Syntheses and Processes | series = ACS Symposium Series | date = 2000 | last1 = Carlson | first1 = Glenn R. | volume = 767 | pages = 8–17 | isbn = 978-0-8412-3678-3 }}</ref> |
||
==See also== |
==See also== |
||
* [[Ecdysone receptor]] |
* [[Ecdysone receptor]] |
||
* [[PTTH]] - Metamorphosis Initiator hormone |
* [[PTTH]] - Metamorphosis Initiator hormone |
||
==References== |
|||
| ⚫ | |||
==External links== |
==External links== |
||
* [ |
* [https://ecdybase.org/ Ecdybase], The Ecdysone Handbook - a free online ecdysteroids database |
||
==Notes== |
|||
| ⚫ | |||
[[Category:Steroids]] |
[[Category:Steroids]] |
||
[[Category:Insect hormones]] |
[[Category:Insect hormones]] |
||
[[Category:Insect developmental biology]] |
[[Category:Insect developmental biology]] |
||
{{insect-stub}} |
|||
Latest revision as of 17:23, 23 August 2024
| |
| |
| Names | |
|---|---|
| IUPAC name
(22R)-2β,3β,14α,22,25-Pentahydroxy-5β-cholest-7-en-6-one
| |
| Systematic IUPAC name
(1R,3aS,5aR,7R,8S,9aR,9bR,11aR)-1-[(2S,3R)-3,6-Dihydroxy-6-methylheptan-2-yl]-3a,7,8-trihydroxy-9a,11a-dimethyl-1,2,3,3a,5a,6,7,8,9,9a,9b,10,11,11a-tetradecahydro-5H-cyclopenta[a]phenanthren-5-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.020.692 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C27H44O6 | |
| Molar mass | 464.63 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ecdysone is a[clarification needed] prohormone of the major insect molting hormone 20-hydroxyecdysone, secreted from the prothoracic glands. It is of steroidal structure. Insect molting hormones (ecdysone and its homologues) are generally called ecdysteroids. Ecdysteroids act as moulting hormones of arthropods but also occur in other related phyla where they can play different roles.[1][2] In Drosophila melanogaster, an increase in ecdysone concentration induces the expression of genes coding for proteins that the larva requires. It causes chromosome puffs (sites of high expression) to form in polytene chromosomes. Recent findings in the laboratory of Chris Q. Doe have found a novel role of this hormone in regulating temporal gene transitions within neural stem cells of the fruit fly.[3]
Ecdysone and other ecdysteroids also appear in many plants mostly as a protection agent (toxins or antifeedants) against herbivorous insects.[4] These phytoecdysteroids have been reputed to have medicinal value. They are part of herbal adaptogenic remedies like Cordyceps, yet an ecdysteroid precursor in plants has been shown to have cytotoxic properties[5] as well as antioxidant properties on lipid peroxidation.[6]
Tebufenozide, sold under the Bayer trademark MIMIC,[7] has ecdysteroid activity although its chemical structure has little resemblance to the ecdysteroids.[8]
See also
[edit]- Ecdysone receptor
- PTTH - Metamorphosis Initiator hormone
References
[edit]- ^ Hoffmeister, Hans; Rufer, Clemens; Ammon, Helmut (February 1, 1965). "Ausscheidung von Ecdyson bei Insekten" [Excretion of Ecdysone by Insects]. Zeitschrift für Naturforschung B. 20 (2): 130–133. doi:10.1515/znb-1965-0207. PMID 14345159. S2CID 95079385. Physiologisch-Chemisches Institut der Universität Marburg/Lahn.
- ^ Karlson, P; Hoffmeister, H (March, 1963). On the biogenesis of ecdyson. I. Conversion of cholesterol into ecdyson. Hoppe Seylers Z Physiol Chem., 331, 289–300. In German. PMID 1396254
- ^ Syed, Mubarak Hussain; Mark, Brandon; Doe, Chris Q. (2017). "Steroid hormone induction of temporal gene expression in Drosophila brain neuroblasts generates neuronal and glial diversity". eLife. 6. doi:10.7554/eLife.26287. PMC 5403213. PMID 28394252.
- ^ Dinan L, Savchenko T, Whiting P (2001). "On the distribution of phytoecdysteroids in plants". Cellular and Molecular Life Sciences. 58 (8): 1121–1132. doi:10.1007/PL00000926. PMC 11337386. PMID 11529504. S2CID 8496934.
- ^ Wang YS, Yang JH, Luo SD, Zhang HB, Li L, Molecules. 2007;12(3):536-42
- ^ Kuzmenko AI, Niki E, Noguchi N (2001). "New functions of 20-hydroxyecdysone in lipid peroxidation". Journal of Oleo Science. 50 (6): 497–506. doi:10.5650/jos.50.497.
- ^ apvma.gov.au: "Tebufenozide in the product Mimic 700 WP Insecticide, Mimic 240 SC Insecticide"
- ^ Carlson, Glenn R. (2000). "Tebufenozide: A Novel Caterpillar Control Agent with Unusually High Target Selectivity". Green Chemical Syntheses and Processes. ACS Symposium Series. Vol. 767. pp. 8–17. doi:10.1021/bk-2000-0767.ch002. ISBN 978-0-8412-3678-3.
External links
[edit]- Ecdybase, The Ecdysone Handbook - a free online ecdysteroids database
