Millerite: Difference between revisions
Chris.urs-o (talk | contribs) added Category:Minerals named after people using HotCat |
modified hatnote |
||
| (39 intermediate revisions by 28 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Nickel sulfide mineral}} |
|||
| ⚫ | |||
{{for|nickel sulfide more generally|Nickel sulfide}} |
|||
| ⚫ | |||
{{for|the neighborhood in Gary|Miller Beach}} |
{{for|the neighborhood in Gary|Miller Beach}} |
||
{{Infobox mineral |
{{Infobox mineral |
||
| Line 5: | Line 7: | ||
| category = [[Sulfide mineral]] |
| category = [[Sulfide mineral]] |
||
| boxwidth = |
| boxwidth = |
||
| image = Millerite |
| image = Millerite in geode (Hall's Gap, Kentucky, USA).jpg |
||
| imagesize = 260px |
|||
| caption = Millerite needles in a quartz geode. Locality: Halls Gap, Lincoln County, Kentucky. Size: 4.0 x 3.5 x 3.0 cm |
|||
| caption = |
|||
| formula = NiS |
| formula = NiS |
||
| IMAsymbol = Mlr<ref>{{Cite journal|last=Warr|first=L.N.|date=2021|title=IMA–CNMNC approved mineral symbols|journal=Mineralogical Magazine|volume=85| issue=3 |pages=291–320| doi=10.1180/mgm.2021.43 | bibcode=2021MinM...85..291W | s2cid=235729616 |doi-access=free}}</ref> |
|||
| strunz = |
| strunz = 2.CC.20 |
||
| symmetry = Trigonal {{overline|3}}2/m |
|||
| |
| system = [[Trigonal]] |
||
| class = Ditrigonal pyramidal (3m) <br/><small>(same [[H-M symbol]])</small> |
|||
| ⚫ | |||
| symmetry = ''R3m'' |
|||
| ⚫ | |||
| unit cell = a = 9.607 Å, c = 3.143 Å; Z = 9 |
|||
| system = [[Trigonal]] Hexagonal Scalenohedral |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| fracture = Uneven |
| fracture = Uneven |
||
| tenacity = Brittle; capillary crystals elastic |
| tenacity = Brittle; capillary crystals elastic |
||
| mohs = |
| mohs = 3–3.5 |
||
| luster = Metallic |
| luster = Metallic |
||
| diaphaneity = Opaque |
| diaphaneity = Opaque |
||
| Line 23: | Line 28: | ||
| pleochroism = |
| pleochroism = |
||
| streak = Greenish black |
| streak = Greenish black |
||
| gravity = 5. |
| gravity = 5.3–5.5 |
||
| melt = |
| melt = |
||
| solubility = |
| solubility = |
||
| other = brittle and becomes magnetic on heating |
| other = brittle and becomes magnetic on heating |
||
| references = <ref name=Handbook>http://rruff.geo.arizona.edu/doclib/hom/millerite.pdf Handbook of Mineralogy</ref><ref name=Mindat>http://www.mindat.org/min-2711.html Mindat</ref><ref name=Webmin>http://webmineral.com/data/Millerite.shtml Webmineral</ref><ref name=Hurlbut>Hurlbut, Cornelius S.; Klein, Cornelis, 1985, Manual of Mineralogy, 20th ed., pp. |
| references = <ref>[https://www.mineralienatlas.de/lexikon/index.php/MineralData?mineral=Millerite Mineralienatlas]</ref><ref name=Handbook>http://rruff.geo.arizona.edu/doclib/hom/millerite.pdf Handbook of Mineralogy</ref><ref name=Mindat>http://www.mindat.org/min-2711.html Mindat</ref><ref name=Webmin>http://webmineral.com/data/Millerite.shtml Webmineral</ref><ref name=Hurlbut>Hurlbut, Cornelius S.; Klein, Cornelis, 1985, Manual of Mineralogy, 20th ed., pp. 279–280, {{ISBN|0-471-80580-7}} |
||
</ref> |
</ref> |
||
}} |
}} |
||
'''Millerite''' is a [[nickel]] [[sulfide mineral]], [[nickel|Ni]][[Sulfur|S]]. It is brassy in colour and has an acicular habit, often forming radiating masses and furry |
'''Millerite''' or ''nickel blende'' is a [[nickel]] [[sulfide mineral]], [[nickel|Ni]][[Sulfur|S]]. It is brassy in colour and has an [[Acicular (crystal habit)|acicular]] habit, often forming radiating masses and furry [[Aggregate (geology)|aggregate]]s. It can be distinguished from pentlandite by crystal habit, its duller colour, and general lack of association with [[pyrite]] or [[pyrrhotite]]. |
||
== Paragenesis == |
== Paragenesis == |
||
Millerite is a common metamorphic mineral replacing [[pentlandite]] within [[serpentinite]] ultramafics. It is formed in this way by removal of sulfur from pentlandite or other nickeliferous sulfide minerals during [[metamorphism]] or [[metasomatism]]. |
Millerite is a common metamorphic mineral replacing [[pentlandite]] within [[serpentinite]] [[Ultramafic rock|ultramafics]]. It is formed in this way by removal of sulfur from pentlandite or other nickeliferous sulfide minerals during [[metamorphism]] or [[metasomatism]]. |
||
Millerite is also formed from sulfur poor [[olivine]] [[cumulate rocks|cumulates]] by nucleation. Millerite is thought to form from sulfur and nickel which exist in pristine olivine in trace amounts, and which are driven out of the olivine during metamorphic processes. [[Magma]]tic olivine generally has up to ~4000 ppm Ni and up to 2500 ppm S within the [[crystal lattice]], as contaminants and substituting for other [[transition metal]]s with similar ionic radii (Fe<sup>2+</sup> and Mn<sup>2+</sup>). |
Millerite is also formed from sulfur poor [[olivine]] [[cumulate rocks|cumulates]] by nucleation. Millerite is thought to form from sulfur and nickel which exist in pristine olivine in trace amounts, and which are driven out of the olivine during metamorphic processes. [[Magma]]tic olivine generally has up to ~4000 ppm Ni and up to 2500 ppm S within the [[crystal lattice]], as contaminants and substituting for other [[transition metal]]s with similar ionic radii (Fe<sup>2+</sup> and Mn<sup>2+</sup>).{{Citation needed|reason=2500 ppm S in crystal lattice is unsupported and mere speculation unless documented|date=February 2018}} |
||
[[Image:Millerite structure.jpg|thumb|left|Millerite structure]] |
[[Image:Millerite structure.jpg|thumb|left|Millerite structure]] |
||
During metamorphism, sulfur and nickel within the olivine lattice are |
During metamorphism, sulfur and nickel within the olivine lattice are reconstituted into metamorphic sulfide minerals, chiefly millerite, during serpentinization and [[talc carbonate]] alteration. When metamorphic olivine is produced, the propensity for this mineral to resorb sulfur, and for the sulfur to be removed via the concomitant loss of volatiles from the serpentinite, tends to lower sulfur [[fugacity]]. |
||
This forms disseminated needle like millerite crystals dispersed throughout the rock mass. |
This forms disseminated needle like millerite crystals dispersed throughout the rock mass. |
||
| Line 51: | Line 56: | ||
== Occurrence == |
== Occurrence == |
||
[[File:Millerite-44389.jpg|thumb|Lustrous mass of intergrown millerite needles from Kalgoorlie, Western Australia |
[[File:Millerite-44389.jpg|thumb|Lustrous mass of intergrown millerite needles from Kalgoorlie, Western Australia (size: 3.9 x 3.5 x 2.2 cm)]] |
||
[[File:Calcite-millerite association.jpg|thumb|Millerite needles partially encased in [[calcite]] and oxidized to [[zaratite]] on their surfaces; from the [[Devonian]] [[Milwaukee Formation]] of [[Wisconsin]]]] |
|||
Millerite is found as a metamorphic replacement of [[pentlandite]] within the Silver Swan nickel deposit, Western Australia, and throughout the many ultramafic serpentinite bodies of the [[Yilgarn craton|Yilgarn Craton]], [[Western Australia]], generally as a replacement of metamorphosed pentlandite. |
Millerite is found as a metamorphic replacement of [[pentlandite]] within the Silver Swan nickel deposit, Western Australia, and throughout the many ultramafic serpentinite bodies of the [[Yilgarn craton|Yilgarn Craton]], [[Western Australia]], generally as a replacement of metamorphosed pentlandite. There is one known occurrence of millerite in South Africa, near Pafuri in the [[Transvaal Province|Transvaal.]] The deposit has never been commercially mined.<ref>{{cite web|title=Millerite|url=https://www.capeminerals.co.za/millerite-2|website=Cape Minerals|access-date=7 February 2017}}</ref> |
||
It is commonly found as radiating clusters of acicular needle-like crystals in cavities in sulfide rich [[limestone]] and [[dolomite]] or in [[geode]]s. It is also found in nickel-iron [[meteorite]]s, such as CK [[carbonaceous chondrites]].<ref>{{cite journal |title=Formation of opaque minerals in CK chondrites |first1=T. |last1=Geiger|first2=A.|last2=Bischoff |
It is commonly found as radiating clusters of acicular needle-like crystals in cavities in sulfide rich [[limestone]] and [[dolomite (rock)|dolomite]] or in [[geode]]s. It is also found in nickel-iron [[meteorite]]s, such as CK [[carbonaceous chondrites]].<ref>{{cite journal |title=Formation of opaque minerals in CK chondrites |first1=T. |last1=Geiger|first2=A.|last2=Bischoff |
||
|doi=10.1016/0032-0633(94)00173-O |journal=Planetary and Space Science |volume=43 |issue=3–4 |year=1995 |pages=485–498 |bibcode = 1995P&SS...43..485G }}</ref> |
|doi=10.1016/0032-0633(94)00173-O |journal=Planetary and Space Science |volume=43 |issue=3–4 |year=1995 |pages=485–498 |bibcode = 1995P&SS...43..485G }}</ref> |
||
Millerite was discovered by [[Wilhelm Haidinger]] in 1845 in the coal mines of [[Wales]]. It was named for British mineralogist [[William Hallowes Miller]]. |
Millerite was discovered by [[Wilhelm Haidinger]] in 1845 in the coal mines of [[Wales]]. It was named for British mineralogist [[William Hallowes Miller]]. The mineral is quite rare in specimen form, and the most common source of the mineral is in the Halls Gap area of [[Lincoln County, Kentucky]] in the [[United States]]. |
||
==See also== |
==See also== |
||
*[[List of minerals]] |
* [[List of minerals]] |
||
*[[List of minerals named after people]] |
* [[List of minerals named after people]] |
||
* [[Nickel Mines, Pennsylvania]] |
|||
==References== |
==References== |
||
| Line 67: | Line 74: | ||
==External links== |
==External links== |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
{{Commonscat}} |
{{Commonscat}} |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
{{sulfur compounds}} |
|||
[[Category:Nickel minerals]] |
[[Category:Nickel minerals]] |
||
[[Category:Sulfide minerals]] |
[[Category:Sulfide minerals]] |
||
[[Category:Trigonal minerals]] |
[[Category:Trigonal minerals]] |
||
[[Category: |
[[Category:Blendes]] |
||
[[Category:Minerals in space group 160]] |
|||
[[ca:Millerita]] |
|||
[[de:Millerit]] |
|||
[[el:Μιλλερίτης]] |
|||
[[es:Millerita]] |
|||
[[eu:Millerita]] |
|||
[[fa:میلریت]] |
|||
[[fr:Millérite]] |
|||
[[it:Millerite]] |
|||
[[he:מילריט]] |
|||
[[nl:Milleriet]] |
|||
[[pl:Milleryt]] |
|||
[[ru:Миллерит]] |
|||
[[fi:Milleriitti]] |
|||
[[sv:Millerit]] |
|||
[[uk:Міллерит]] |
|||
[[vi:Millerit]] |
|||
Latest revision as of 14:19, 24 August 2024
| Millerite | |
|---|---|
 | |
| General | |
| Category | Sulfide mineral |
| Formula (repeating unit) | NiS |
| IMA symbol | Mlr[1] |
| Strunz classification | 2.CC.20 |
| Crystal system | Trigonal |
| Crystal class | Ditrigonal pyramidal (3m) (same H-M symbol) |
| Space group | R3m |
| Unit cell | a = 9.607 Å, c = 3.143 Å; Z = 9 |
| Identification | |
| Colour | Pale brass-yellow to bronze-yellow, tarnishes to iridescence |
| Crystal habit | Typically acicular (needle-like) often in radial sprays – also massive |
| Cleavage | Perfect on {1011} and {0112} – obscured by typical form |
| Fracture | Uneven |
| Tenacity | Brittle; capillary crystals elastic |
| Mohs scale hardness | 3–3.5 |
| Luster | Metallic |
| Streak | Greenish black |
| Diaphaneity | Opaque |
| Specific gravity | 5.3–5.5 |
| Other characteristics | brittle and becomes magnetic on heating |
| References | [2][3][4][5][6] |
Millerite or nickel blende is a nickel sulfide mineral, NiS. It is brassy in colour and has an acicular habit, often forming radiating masses and furry aggregates. It can be distinguished from pentlandite by crystal habit, its duller colour, and general lack of association with pyrite or pyrrhotite.
Paragenesis
[edit]Millerite is a common metamorphic mineral replacing pentlandite within serpentinite ultramafics. It is formed in this way by removal of sulfur from pentlandite or other nickeliferous sulfide minerals during metamorphism or metasomatism.
Millerite is also formed from sulfur poor olivine cumulates by nucleation. Millerite is thought to form from sulfur and nickel which exist in pristine olivine in trace amounts, and which are driven out of the olivine during metamorphic processes. Magmatic olivine generally has up to ~4000 ppm Ni and up to 2500 ppm S within the crystal lattice, as contaminants and substituting for other transition metals with similar ionic radii (Fe2+ and Mn2+).[citation needed]
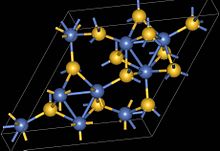
During metamorphism, sulfur and nickel within the olivine lattice are reconstituted into metamorphic sulfide minerals, chiefly millerite, during serpentinization and talc carbonate alteration. When metamorphic olivine is produced, the propensity for this mineral to resorb sulfur, and for the sulfur to be removed via the concomitant loss of volatiles from the serpentinite, tends to lower sulfur fugacity.
This forms disseminated needle like millerite crystals dispersed throughout the rock mass.
Millerite may be associated with heazlewoodite and is considered a transitional stage in the metamorphic production of heazlewoodite via the above process.
Economic importance
[edit]Millerite, when found in enough concentration, is a very important ore of nickel because, for its mass as a sulfide mineral, it contains a higher percentage of nickel than pentlandite. This means that, for every percent of millerite, an ore contains more nickel than an equivalent percentage of pentlandite sulfide.
Millerite forms an important ore constituent of the Silver Swan, Wannaway, Cliffs, Honeymoon Well, Yakabindie and Mt Keith (MKD5) orebodies. It is an accessory mineral associated with nickel laterite deposits in New Caledonia.
Occurrence
[edit]

Millerite is found as a metamorphic replacement of pentlandite within the Silver Swan nickel deposit, Western Australia, and throughout the many ultramafic serpentinite bodies of the Yilgarn Craton, Western Australia, generally as a replacement of metamorphosed pentlandite. There is one known occurrence of millerite in South Africa, near Pafuri in the Transvaal. The deposit has never been commercially mined.[7]
It is commonly found as radiating clusters of acicular needle-like crystals in cavities in sulfide rich limestone and dolomite or in geodes. It is also found in nickel-iron meteorites, such as CK carbonaceous chondrites.[8]
Millerite was discovered by Wilhelm Haidinger in 1845 in the coal mines of Wales. It was named for British mineralogist William Hallowes Miller. The mineral is quite rare in specimen form, and the most common source of the mineral is in the Halls Gap area of Lincoln County, Kentucky in the United States.
See also
[edit]References
[edit]- ^ Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- ^ Mineralienatlas
- ^ http://rruff.geo.arizona.edu/doclib/hom/millerite.pdf Handbook of Mineralogy
- ^ http://www.mindat.org/min-2711.html Mindat
- ^ http://webmineral.com/data/Millerite.shtml Webmineral
- ^ Hurlbut, Cornelius S.; Klein, Cornelis, 1985, Manual of Mineralogy, 20th ed., pp. 279–280, ISBN 0-471-80580-7
- ^ "Millerite". Cape Minerals. Retrieved 7 February 2017.
- ^ Geiger, T.; Bischoff, A. (1995). "Formation of opaque minerals in CK chondrites". Planetary and Space Science. 43 (3–4): 485–498. Bibcode:1995P&SS...43..485G. doi:10.1016/0032-0633(94)00173-O.
