Cyanogen bromide: Difference between revisions
m Added FDA UNII |
nucleophilic acyl substitution issue |
||
| (30 intermediate revisions by 19 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Chemical compound (BrCN)}} |
|||
{{redirect-distinguish|CBrN|CBRN}} |
{{redirect-distinguish|CBrN|CBRN}} |
||
{{Chembox |
{{Chembox |
||
| |
|Watchedfields = changed |
||
| |
|verifiedrevid = 442343462 |
||
| ⚫ | |||
| Name = |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |PIN = Carbononitridic bromide<ref>{{Cite web|title=Cyanogen Bromide – Compound Summary |url=https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=10476#x291|work=PubChem Compound|publisher=National Center for Biotechnology Information|access-date=4 June 2012|location=USA|date=26 March 2005|at=Identification}}</ref> |
||
| ⚫ | |||
| ⚫ | |OtherNames = {{Unbulleted list|Bromine cyanide<ref name="Merck">{{cite book |title=The Merck Index|edition=10th|year=1983|publisher=Merck & Co. |location=Rahway, NJ|page=385}}</ref>|Campilit<ref>{{cite web|url=http://www.chemindustry.com/chemicals/0308894.html|title=Campilit, CAS Number: 506-68-3|access-date=2013-03-14|archive-date=2023-03-20|archive-url=https://web.archive.org/web/20230320080339/https://www.chemindustry.com/chemicals/0308894.html|url-status=dead}}</ref>| |
||
| ⚫ | | |
||
| SystematicName = |
|||
| ⚫ | | |
||
}} |
}} |
||
| ⚫ | |||
| IUPACName = |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| Abbreviations = |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
|RTECS = GT2100000 |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| |
|SMILES = BrC#N |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| SMILES = BrC#N |
|||
| ⚫ | |||
| ⚫ | |||
| |
|StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
||
| ⚫ | |||
| ⚫ | |||
}} |
}} |
||
| |
|Section2 = {{Chembox Properties |
||
| |
|Formula = BrCN |
||
| |
|MolarMass = 105.921 g mol<sup>−1</sup> |
||
| |
|Appearance = Colorless solid |
||
| |
|Density = 2.015 g mL<sup>−1</sup> |
||
| |
|MeltingPtC = 50 to 53 |
||
| |
|BoilingPtC = 61 to 62 |
||
| |
|Solubility = Reacts |
||
| |
|VaporPressure = 16.2 kPa |
||
}} |
}} |
||
| |
|Section3 = {{Chembox Thermochemistry |
||
| |
|DeltaHf = 136.1–144.7 kJ mol<sup>−1</sup> |
||
}} |
}} |
||
| |
|Section4 = {{Chembox Hazards |
||
| |
|GHSPictograms = {{GHS corrosion}} {{GHS skull and crossbones}} {{GHS environment}} |
||
| |
|GHSSignalWord = '''DANGER''' |
||
| |
|HPhrases = {{H-phrases|300|310|314|330|410}} |
||
| |
|PPhrases = {{P-phrases|260|273|280|284|302+350}} |
||
| |
|NFPA-H = 4 |
||
| |
|NFPA-F = 0 |
||
| |
|NFPA-R = 1 |
||
| |
|PEL = 5 mg m<sup>−3</sup> |
||
}} |
}} |
||
| Section5 = {{Chembox Related |
| Section5 = {{Chembox Related |
||
| Line 64: | Line 61: | ||
| OtherFunction = {{Unbulleted list|[[Hydrogen cyanide]]|[[Thiocyanic acid]]|[[Cyanogen iodide]]|[[Cyanogen chloride]]|[[Cyanogen fluoride]]|[[Acetonitrile]]|[[Aminoacetonitrile]]|[[Glycolonitrile]]|[[Cyanogen]]}} |
| OtherFunction = {{Unbulleted list|[[Hydrogen cyanide]]|[[Thiocyanic acid]]|[[Cyanogen iodide]]|[[Cyanogen chloride]]|[[Cyanogen fluoride]]|[[Acetonitrile]]|[[Aminoacetonitrile]]|[[Glycolonitrile]]|[[Cyanogen]]}} |
||
}} |
}} |
||
| Section6 = |
|||
}} |
}} |
||
'''Cyanogen bromide''' is the [[inorganic compound]] with the [[chemical formula|formula]] (CN)Br or |
|||
BrCN. It is a colorless solid that is widely used to modify [[biopolymer]]s, fragment [[protein]]s and [[peptide]]s (cuts the C-terminus of methionine), and synthesize other compounds. |
'''Cyanogen bromide''' is the [[inorganic compound]] with the [[chemical formula|formula]] (CN)Br or BrCN. It is a colorless solid that is widely used to modify [[biopolymer]]s, fragment [[protein]]s and [[peptide]]s (cuts the C-terminus of methionine), and synthesize other compounds. The compound is classified as a [[pseudohalogen]]. |
||
== Synthesis, basic properties, and structure == |
== Synthesis, basic properties, and structure == |
||
The [[carbon]] atom in cyanogen bromide is bonded to [[bromine]] by a single bond and to [[nitrogen]] by a [[triple bond]] (i.e. |
The [[carbon]] atom in cyanogen bromide is bonded to [[bromine]] by a single bond and to [[nitrogen]] by a [[triple bond]] (i.e. {{chem2|Br\sC\tN}}). The compound is linear and polar, but it does not spontaneously ionize in water. It dissolves in both water and polar [[organic solvent]]s. |
||
Cyanogen bromide can be prepared by [[oxidation]] of [[sodium cyanide]] with [[bromine]], which proceeds in two steps via the intermediate [[cyanogen]] ((CN) |
Cyanogen bromide can be prepared by [[oxidation]] of [[sodium cyanide]] with [[bromine]], which proceeds in two steps via the intermediate [[cyanogen]] ({{chem2|(CN)2}}): |
||
:2 NaCN |
:<chem>2 NaCN + Br2 -> (CN)2 + 2 NaBr</chem> |
||
:(CN) |
:<chem>(CN)2 + Br2 -> 2 (CN)Br</chem> |
||
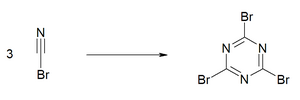
When refrigerated the material has an extended shelflife. Like some other cyanogen compounds, cyanogen bromide undergoes an exothermic trimerisation to [[cyanuric bromide]] ((BrCN) |
When refrigerated the material has an extended shelflife. Like some other cyanogen compounds, cyanogen bromide undergoes an exothermic trimerisation to [[cyanuric bromide]] ({{chem2|(BrCN)3}}). This reaction is catalyzed by traces of bromine, metal salts, acids and bases. For this reason, experimentalists avoid brownish samples.<ref name=EROS/> |
||
:[[File:Cyanuric bromide synthesis.PNG|300px]] |
:[[File:Cyanuric bromide synthesis.PNG|300px]] |
||
Cyanogen bromide is [[hydrolyzed]] to |
Cyanogen bromide is [[hydrolyzed]] to form [[hydrogen cyanate]] and [[hydrobromic acid]]: <chem display=block>(CN)Br + H2O -> HOCN + HBr</chem> |
||
:(CN)Br + H<sub>2</sub>O → HCN + HOBr |
|||
== Biochemical applications == |
== Biochemical applications == |
||
| Line 90: | Line 85: | ||
=== Protein immobilization === |
=== Protein immobilization === |
||
Cyanogen bromide is often used to immobilize proteins by coupling them to [[reagent]]s such as [[agarose]] for [[affinity chromatography]].<ref name="immobilized">{{ |
Cyanogen bromide is often used to immobilize proteins by coupling them to [[reagent]]s such as [[agarose]] for [[affinity chromatography]].<ref name="immobilized">{{cite book | title = Immobilized Affinity Ligand Techniques |author1=Hermanson, G. T. |author2=Mallia, A. K. |author3=Smith, P. K. | publisher = Academic Press | year = 1992 | isbn = 978-0-12-342330-6}}</ref> Because of its simplicity and mild [[pH]] conditions, cyanogen bromide activation is the most common method for preparing affinity gels. Cyanogen bromide is also often used because it reacts with the [[hydroxyl]] groups on agarose to form [[cyanate]] [[ester]]s and [[imidocarbonate]]s. These groups are reacted with [[primary amine]]s in order to couple the protein onto the agarose matrix, as shown in the figure. Because cyanate esters are more reactive than are cyclic imidocarbonates, the amine will react mostly with the ester, yielding [[isourea]] derivatives, and partially with the less reactive imidocarbonate, yielding substituted imidocarbonates.<ref name="sigma">{{cite web | url = http://www.sigmaaldrich.com/etc/medialib/docs/Sigma/Product_Information_Sheet/c9210pis.pdf | title = Cyanogen Bromide Activated Matrices | publisher = Sigma}} {{dead link|date=June 2020}}</ref> |
||
The disadvantages of this approach include the toxicity of cyanogen bromide and its sensitivity to oxidation. Also, cyanogen bromide activation involves the attachment of a [[ligand]] to agarose by an isourea bond, which is positively charged at neutral pH and thus unstable. Consequently, isourea derivatives may act as weak [[anion exchanger]]s.<ref name="sigma" |
The disadvantages of this approach include the toxicity of cyanogen bromide and its sensitivity to oxidation. Also, cyanogen bromide activation involves the attachment of a [[ligand]] to agarose by an isourea bond, which is positively charged at neutral pH and thus unstable. Consequently, isourea derivatives may act as weak [[anion exchanger]]s.<ref name="sigma"/>{{dead link|date=June 2020}} |
||
=== Protein cleavage === |
=== Protein cleavage === |
||
| Line 100: | Line 95: | ||
[[File:CNBr5.png|thumb|left|150px|Cyanogen bromide peptide bond cleavage]] |
[[File:CNBr5.png|thumb|left|150px|Cyanogen bromide peptide bond cleavage]] |
||
The [[electron density]] in cyanogen bromide is shifted away from the carbon atom, making it unusually [[electrophilic]], and towards the more [[electronegative]] bromine and nitrogen. This leaves the carbon particularly vulnerable to attack by a [[nucleophile]], and the cleavage reaction begins with a |
The [[electron density]] in cyanogen bromide is shifted away from the carbon atom, making it unusually [[electrophilic]], and towards the more [[electronegative]] bromine and nitrogen. This leaves the carbon particularly vulnerable to attack by a [[nucleophile]], and the cleavage reaction begins with a substitution reaction in which bromine is ultimately replaced by the sulfur in methionine. This attack is followed by the formation of a five-membered ring as opposed to a six-membered ring, which would entail the formation of a [[double bond]] in the ring between nitrogen and carbon. This double bond would result in a rigid ring conformation, thereby destabilizing the molecule. Thus, the five-membered ring is formed so that the double bond is outside the ring, as shown in the figure. |
||
Although the nucleophilic sulfur in methionine is responsible for attacking BrCN, the sulfur in [[cysteine]] does not behave similarly. If the sulfur in cysteine attacked cyanogen bromide, the bromide ion would deprotonate the cyanide [[adduct]], leaving the sulfur uncharged and the beta carbon of the cysteine not electrophilic. The strongest electrophile would then be the cyanide |
Although the nucleophilic sulfur in methionine is responsible for attacking BrCN, the sulfur in [[cysteine]] does not behave similarly. If the sulfur in cysteine attacked cyanogen bromide, the bromide ion would deprotonate the cyanide [[adduct]], leaving the sulfur uncharged and the beta carbon of the cysteine not electrophilic. The strongest electrophile would then be the cyanide carbon, which, if attacked by water, would yield [[cyanic acid]] and the original cysteine. |
||
==== Reaction conditions ==== |
==== Reaction conditions ==== |
||
Cleaving proteins with BrCN requires using a [[buffer solution|buffer]] such as 0.1M HCl ([[hydrochloric acid]]) or 70% ([[formic acid]]).<ref>{{ |
Cleaving proteins with BrCN requires using a [[buffer solution|buffer]] such as 0.1M HCl ([[hydrochloric acid]]) or 70% ([[formic acid]]).<ref>{{cite journal |author1=Schroeder, W. A. |author2=Shelton, J. B. |author3=Shelton, J. R. | title = An Examination of Conditions for the Cleavage of Polypeptide Chains with Cyanogen Bromide | journal = Archives of Biochemistry and Biophysics | year = 1969 | volume = 130 | issue = 1 | pages = 551–556 | doi = 10.1016/0003-9861(69)90069-1 |pmid=5778667}}</ref> These are the most common buffers for cleavage. An advantage to HCl is that formic acid causes the formation of formyl esters, which complicates protein characterization. However, formic is still often used because it dissolves most proteins. Also, the oxidation of methionine to [[methionine sulfoxide]], which is inert to BrCN attack, occurs more readily in HCl than in formic acid, possibly because formic acid is a reducing acid. Alternative buffers for cleavage include [[guanidine]] or [[urea]] in HCl because of their ability to [[protein folding|unfold proteins]], thereby making methionine more accessible to BrCN.<ref name="kaiser">{{ cite journal |author1=Kaiser, R. |author2=Metzka, L. | title = Enhancement of Cyanogen Bromide Cleavage Yields for Methionyl-Serine and Methionyl-Threonine Peptide Bonds | journal = Analytical Biochemistry | year = 1999 | volume = 266 | issue = 1 | pages = 1–8 | doi = 10.1006/abio.1998.2945 | pmid=9887207}}</ref> |
||
Water is required for normal peptide bond cleavage of the [[iminolactone]] intermediate. In formic acid, cleavage of Met-[[serine|Ser]] and Met-[[threonine|Thr]] bonds is enhanced with increased water concentration because these conditions favor the addition of water across the [[imine]] rather than reaction of the side chain hydroxyl with the imine. Lowered pH tends to increase cleavage rates by inhibiting methionine side chain oxidation.<ref name="kaiser" /> |
|||
==== Side reactions ==== |
==== Side reactions ==== |
||
| Line 113: | Line 108: | ||
== Organic synthesis == |
== Organic synthesis == |
||
Cyanogen bromide is a common reagent in [[organic synthesis]]. In most reactions, it acts as a source of electrophilic [[cyanide|cyanogen]] and nucleophilic [[bromide]]; carbocations preferentially attack the nitrogen atom.<ref name=EROS/> In the presence of a Lewis acid, it cyanidates [[arene]]s.<ref name=SynLett/> |
|||
Cyanogen bromide is a common reagent in [[organic synthesis]].<ref name=EROS>{{cite journal|title=Cyanogen Bromide|author1=Joel Morris|author2= Lajos Kovács|journal=Encyclopedia of Reagents for Organic Synthesis|year=2008|doi=10.1002/047084289X.rc269.pub2|isbn=978-0471936237}}</ref> As stated earlier, the reagent is prone to attack by nucleophiles such as amines and alcohols because of the electrophilic carbon. In the synthesis of [[cyanamide]]s and [[dicyanamide]]s, primary and secondary amines react with BrCN to yield mono- and dialkylcyanamides, which can further react with amines and [[hydroxylamine]] to yield [[guanidine]]s and [[hydroxyguanidine]]s. In the [[von Braun reaction]], tertiary amines react with BrCN to yield disubstituted cyanamides and an alkyl bromide. Cyanogen bromide can be used to prepare [[aryl]] [[nitrile]]s, nitriles, [[anhydride]]s, and [[cyanate]]s. It can also serve as a cleaving agent.<ref>{{ cite journal | author = Kumar, V. | title = Cyanogen Bromide (CNBr) | journal = Synlett | year = 2005 | volume = 2005 | issue = 10 | pages = 1638–1639 | doi = 10.1055/s-2005-869872 | url = http://www.thieme-connect.com/ejournals/pdf/synlett/doi/10.1055/s-2005-869872.pdf | id = Art ID: V12705ST }}</ref> |
|||
| ⚫ | |||
BrCN converts [[alcohols]] to [[cyanates]]; [[amine]]s to [[cyanamide]]s or [[dicyanamide]]s.<ref name=EROS>{{cite encyclopedia|entry=Cyanogen Bromide|author1=Joel Morris|author2= Lajos Kovács|author3=Kouichi Ohe|encyclopedia=Encyclopedia of Reagents for Organic Synthesis|year=2015|doi=10.1002/047084289X.rc269.pub3|isbn=978-0471936237}}</ref> Excess BrCN continues the reaction to [[guanidine]]s; [[hydroxylamine]]s yield [[hydroxyguanidine]]s similarly.<ref name=SynLett/> |
|||
The cyanamides so formed [[umpolung|umpole]] the original amine, and tends to eliminate alkyl substituents. In the [[von Braun reaction]], tertiary amines react with cyanogen bromide to yield disubstituted cyanamides and an alkyl bromide.<ref name=SynLett>{{ cite journal | author = Kumar, V. | title = Cyanogen Bromide (CNBr) | journal = Synlett | year = 2005 | volume = 2005 | issue = 10 | pages = 1638–1639 | doi = 10.1055/s-2005-869872 | url = http://www.thieme-connect.com/ejournals/pdf/synlett/doi/10.1055/s-2005-869872.pdf | id = Art ID: V12705ST | doi-access = free }}</ref> That net reaction is similar to the [[Polonovski reaction|Polonovski elimination]], but does not require N-oxidation.<ref name=EROS/> |
|||
In bromocyanation, BrCN adds across multiple bonds to give a vicinal cyanobromide. Bromocyanated [[enol]]s spontaneously undergo a [[Darzens reaction|Darzens-like elimination]] to an epoxynitrile.<ref name=EROS/> |
|||
Cyanogen bromide is also a dehydrating agent, hydrolyzing to [[hydrogen bromide]] and [[cyanic acid]].<ref name=SynLett /> |
|||
| ⚫ | |||
== Toxicity, storage, and deactivation == |
== Toxicity, storage, and deactivation == |
||
Cyanogen bromide can be stored under dry conditions at 2 to 8 °C for extended periods.<ref name="sigma" /> |
Cyanogen bromide can be stored under dry conditions at 2 to 8 °C for extended periods.<ref name="sigma" /> |
||
Cyanogen bromide is volatile, and readily absorbed through the [[skin]] or [[gastrointestinal tract]]. Therefore, toxic exposure may occur by inhalation, physical contact, or ingestion. It is acutely toxic, causing a variety of [[nonspecific symptoms]]. Exposure to even small amounts may cause convulsions or death. LD<sub>50</sub> orally in rats is reported as 25–50 mg/kg.<ref name="NIH">{{ |
Cyanogen bromide is volatile, and readily absorbed through the [[skin]] or [[gastrointestinal tract]]. Therefore, toxic exposure may occur by inhalation, physical contact, or ingestion. It is acutely toxic, causing a variety of [[nonspecific symptoms]]. Exposure to even small amounts may cause convulsions or death. LD<sub>50</sub> orally in rats is reported as 25–50 mg/kg.<ref name="NIH">{{cite web | url = http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+708 | title = Cyanogen Bromide HSDB 708 | work = HSDB | publisher = NIH / NLM | date = 2009-04-07}}</ref> |
||
The recommended method to deactivate cyanogen bromide is with [[bleach]].<ref>{{ cite journal |author1=Lunn, G. |author2=Sansone, E. B. | title = Destruction of Cyanogen Bromide and Inorganic Cyanides | journal = [[Analytical Biochemistry (journal)|Analytical Biochemistry]] | year = 1985 | volume = 147 | issue = 1 | pages = 245–250 | doi = 10.1016/0003-2697(85)90034-X | pmid = 4025821 }}</ref> The aqueous alkali hydroxide instantly hydrolyzes (CN)Br to alkali cyanide and bromide. The cyanide can then be oxidized by [[sodium hypochlorite|sodium]] or [[calcium hypochlorite]] to the less toxic cyanate ion. |
The recommended method to deactivate cyanogen bromide is with [[sodium hydroxide]] and [[bleach]].<ref>{{ cite journal |author1=Lunn, G. |author2=Sansone, E. B. | title = Destruction of Cyanogen Bromide and Inorganic Cyanides | journal = [[Analytical Biochemistry (journal)|Analytical Biochemistry]] | year = 1985 | volume = 147 | issue = 1 | pages = 245–250 | doi = 10.1016/0003-2697(85)90034-X | pmid = 4025821 |url=https://zenodo.org/record/1253784 }}</ref> The aqueous alkali hydroxide instantly hydrolyzes (CN)Br to alkali cyanide and bromide. The cyanide can then be oxidized by [[sodium hypochlorite|sodium]] or [[calcium hypochlorite]] to the less toxic cyanate ion. Deactivation is extremely [[exothermic]] and may be explosive.<ref name="NIH" /> |
||
== References == |
== References == |
||
| Line 127: | Line 131: | ||
== Further reading == |
== Further reading == |
||
* {{ cite journal |author1=Gross, E. |author2=Witkop, B. | title = Nonenzymatic Cleavage of Peptide Bonds: The Methionine Residues in Bovine Pancreatic Ribonuclease | journal = [[Journal of Biological Chemistry]] | year = 1962 | volume = 237 | issue = 6 | pages = 1856–1860 | pmid = 13902203 | url = http://www.jbc.org/content/237/6/1856.full.pdf }} |
* {{ cite journal |author1=Gross, E. |author2=Witkop, B. | title = Nonenzymatic Cleavage of Peptide Bonds: The Methionine Residues in Bovine Pancreatic Ribonuclease | journal = [[Journal of Biological Chemistry]] | year = 1962 | volume = 237 | issue = 6 | pages = 1856–1860 |doi=10.1016/S0021-9258(19)73948-9 | pmid = 13902203 | url = http://www.jbc.org/content/237/6/1856.full.pdf |doi-access=free }} |
||
* {{ cite journal | author1 = Inglis, A. S. | author2 = Edman, P. | |
* {{ cite journal | author1 = Inglis, A. S. | author2 = Edman, P. | author-link2 = Pehr Edman | title = Mechanism of Cyanogen Bromide Reaction with Methionine in Peptides and Proteins | journal = [[Analytical Biochemistry (journal)|Analytical Biochemistry]] | year = 1970 | volume = 37 | issue = 1 | pages = 73–80 | doi = 10.1016/0003-2697(70)90259-9 | pmid = 5506566 }} |
||
== External links == |
== External links == |
||
* {{ cite web | url = http://hazard.com/msds/mf/baker/baker/files/c6600.htm | title = Cyanogen Bromide MSDS Number: C6600 | date = 1996-08-12 | publisher = J. T. Baker }} |
* {{ cite web | url = http://hazard.com/msds/mf/baker/baker/files/c6600.htm | title = Cyanogen Bromide MSDS Number: C6600 | date = 1996-08-12 | publisher = J. T. Baker }} |
||
* {{ |
* {{cite journal | author = Teeri, A. E. | title = Thiamine and the Cyanogen Bromide Reaction | journal = Journal of Biological Chemistry | year = 1948 | volume = 173 | issue = 2 | pages = 503–505 | doi = 10.1016/S0021-9258(18)57422-6 | pmid = 18910706 | doi-access = free }} |
||
{{Chemical agents}} |
|||
{{Cyanides}} |
{{Cyanides}} |
||
[[Category: |
[[Category:Bromine compounds]] |
||
[[Category: |
[[Category:Triatomic molecules]] |
||
[[Category: |
[[Category:Cyano compounds]] |
||
[[Category:Nonmetal halides]] |
[[Category:Nonmetal halides]] |
||
[[Category:Blood agents]] |
|||
[[Category:Lachrymatory agents]] |
|||
[[Category:Pseudohalogens]] |
|||
Latest revision as of 13:24, 26 August 2024
| |
| Names | |
|---|---|
| Preferred IUPAC name
Carbononitridic bromide[3] | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| 1697296 | |
| ChemSpider | |
| ECHA InfoCard | 100.007.320 |
| EC Number |
|
| MeSH | Cyanogen+Bromide |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1889 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| BrCN | |
| Molar mass | 105.921 g mol−1 |
| Appearance | Colorless solid |
| Density | 2.015 g mL−1 |
| Melting point | 50 to 53 °C (122 to 127 °F; 323 to 326 K) |
| Boiling point | 61 to 62 °C (142 to 144 °F; 334 to 335 K) |
| Reacts | |
| Vapor pressure | 16.2 kPa |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
136.1–144.7 kJ mol−1 |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H300, H310, H314, H330, H410 | |
| P260, P273, P280, P284, P302+P350 | |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
5 mg m−3 |
| Related compounds | |
Related alkanenitriles
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides (cuts the C-terminus of methionine), and synthesize other compounds. The compound is classified as a pseudohalogen.
Synthesis, basic properties, and structure
[edit]The carbon atom in cyanogen bromide is bonded to bromine by a single bond and to nitrogen by a triple bond (i.e. Br−C≡N). The compound is linear and polar, but it does not spontaneously ionize in water. It dissolves in both water and polar organic solvents.
Cyanogen bromide can be prepared by oxidation of sodium cyanide with bromine, which proceeds in two steps via the intermediate cyanogen ((CN)2):
When refrigerated the material has an extended shelflife. Like some other cyanogen compounds, cyanogen bromide undergoes an exothermic trimerisation to cyanuric bromide ((BrCN)3). This reaction is catalyzed by traces of bromine, metal salts, acids and bases. For this reason, experimentalists avoid brownish samples.[4]
Cyanogen bromide is hydrolyzed to form hydrogen cyanate and hydrobromic acid:
Biochemical applications
[edit]The main uses of cyanogen bromide are to immobilize proteins, fragment proteins by cleaving peptide bonds, and synthesize cyanamides and other molecules.

Protein immobilization
[edit]Cyanogen bromide is often used to immobilize proteins by coupling them to reagents such as agarose for affinity chromatography.[5] Because of its simplicity and mild pH conditions, cyanogen bromide activation is the most common method for preparing affinity gels. Cyanogen bromide is also often used because it reacts with the hydroxyl groups on agarose to form cyanate esters and imidocarbonates. These groups are reacted with primary amines in order to couple the protein onto the agarose matrix, as shown in the figure. Because cyanate esters are more reactive than are cyclic imidocarbonates, the amine will react mostly with the ester, yielding isourea derivatives, and partially with the less reactive imidocarbonate, yielding substituted imidocarbonates.[6]
The disadvantages of this approach include the toxicity of cyanogen bromide and its sensitivity to oxidation. Also, cyanogen bromide activation involves the attachment of a ligand to agarose by an isourea bond, which is positively charged at neutral pH and thus unstable. Consequently, isourea derivatives may act as weak anion exchangers.[6][dead link]
Protein cleavage
[edit]Cyanogen bromide hydrolyzes peptide bonds at the C-terminus of methionine residues. This reaction is used to reduce the size of polypeptide segments for identification and sequencing.
Mechanism
[edit]
The electron density in cyanogen bromide is shifted away from the carbon atom, making it unusually electrophilic, and towards the more electronegative bromine and nitrogen. This leaves the carbon particularly vulnerable to attack by a nucleophile, and the cleavage reaction begins with a substitution reaction in which bromine is ultimately replaced by the sulfur in methionine. This attack is followed by the formation of a five-membered ring as opposed to a six-membered ring, which would entail the formation of a double bond in the ring between nitrogen and carbon. This double bond would result in a rigid ring conformation, thereby destabilizing the molecule. Thus, the five-membered ring is formed so that the double bond is outside the ring, as shown in the figure.
Although the nucleophilic sulfur in methionine is responsible for attacking BrCN, the sulfur in cysteine does not behave similarly. If the sulfur in cysteine attacked cyanogen bromide, the bromide ion would deprotonate the cyanide adduct, leaving the sulfur uncharged and the beta carbon of the cysteine not electrophilic. The strongest electrophile would then be the cyanide carbon, which, if attacked by water, would yield cyanic acid and the original cysteine.
Reaction conditions
[edit]Cleaving proteins with BrCN requires using a buffer such as 0.1M HCl (hydrochloric acid) or 70% (formic acid).[7] These are the most common buffers for cleavage. An advantage to HCl is that formic acid causes the formation of formyl esters, which complicates protein characterization. However, formic is still often used because it dissolves most proteins. Also, the oxidation of methionine to methionine sulfoxide, which is inert to BrCN attack, occurs more readily in HCl than in formic acid, possibly because formic acid is a reducing acid. Alternative buffers for cleavage include guanidine or urea in HCl because of their ability to unfold proteins, thereby making methionine more accessible to BrCN.[8]
Water is required for normal peptide bond cleavage of the iminolactone intermediate. In formic acid, cleavage of Met-Ser and Met-Thr bonds is enhanced with increased water concentration because these conditions favor the addition of water across the imine rather than reaction of the side chain hydroxyl with the imine. Lowered pH tends to increase cleavage rates by inhibiting methionine side chain oxidation.[8]
Side reactions
[edit]When methionine is followed by serine or threonine, side reactions can occur that destroy the methionine without peptide bond cleavage. Normally, once the iminolactone is formed (refer to figure), water and acid can react with the imine to cleave the peptide bond, forming a homoserine lactone and new C-terminal peptide. However, if the adjacent amino acid to methionine has a hydroxyl or sulfhydryl group, this group can react with the imine to form a homoserine without peptide bond cleavage.[8] These two cases are shown in the figure.
Organic synthesis
[edit]Cyanogen bromide is a common reagent in organic synthesis. In most reactions, it acts as a source of electrophilic cyanogen and nucleophilic bromide; carbocations preferentially attack the nitrogen atom.[4] In the presence of a Lewis acid, it cyanidates arenes.[9]
BrCN converts alcohols to cyanates; amines to cyanamides or dicyanamides.[4] Excess BrCN continues the reaction to guanidines; hydroxylamines yield hydroxyguanidines similarly.[9]
The cyanamides so formed umpole the original amine, and tends to eliminate alkyl substituents. In the von Braun reaction, tertiary amines react with cyanogen bromide to yield disubstituted cyanamides and an alkyl bromide.[9] That net reaction is similar to the Polonovski elimination, but does not require N-oxidation.[4]
In bromocyanation, BrCN adds across multiple bonds to give a vicinal cyanobromide. Bromocyanated enols spontaneously undergo a Darzens-like elimination to an epoxynitrile.[4]
Cyanogen bromide is also a dehydrating agent, hydrolyzing to hydrogen bromide and cyanic acid.[9]
The compound is used in the synthesis of 4-methylaminorex ("ice") and viroxime.
Toxicity, storage, and deactivation
[edit]Cyanogen bromide can be stored under dry conditions at 2 to 8 °C for extended periods.[6]
Cyanogen bromide is volatile, and readily absorbed through the skin or gastrointestinal tract. Therefore, toxic exposure may occur by inhalation, physical contact, or ingestion. It is acutely toxic, causing a variety of nonspecific symptoms. Exposure to even small amounts may cause convulsions or death. LD50 orally in rats is reported as 25–50 mg/kg.[10]
The recommended method to deactivate cyanogen bromide is with sodium hydroxide and bleach.[11] The aqueous alkali hydroxide instantly hydrolyzes (CN)Br to alkali cyanide and bromide. The cyanide can then be oxidized by sodium or calcium hypochlorite to the less toxic cyanate ion. Deactivation is extremely exothermic and may be explosive.[10]
References
[edit]- ^ The Merck Index (10th ed.). Rahway, NJ: Merck & Co. 1983. p. 385.
- ^ "Campilit, CAS Number: 506-68-3". Archived from the original on 2023-03-20. Retrieved 2013-03-14.
- ^ "Cyanogen Bromide – Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 26 March 2005. Identification. Retrieved 4 June 2012.
- ^ a b c d e Joel Morris; Lajos Kovács; Kouichi Ohe (2015). "Cyanogen Bromide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rc269.pub3. ISBN 978-0471936237.
- ^ Hermanson, G. T.; Mallia, A. K.; Smith, P. K. (1992). Immobilized Affinity Ligand Techniques. Academic Press. ISBN 978-0-12-342330-6.
- ^ a b c "Cyanogen Bromide Activated Matrices" (PDF). Sigma. [dead link]
- ^ Schroeder, W. A.; Shelton, J. B.; Shelton, J. R. (1969). "An Examination of Conditions for the Cleavage of Polypeptide Chains with Cyanogen Bromide". Archives of Biochemistry and Biophysics. 130 (1): 551–556. doi:10.1016/0003-9861(69)90069-1. PMID 5778667.
- ^ a b c Kaiser, R.; Metzka, L. (1999). "Enhancement of Cyanogen Bromide Cleavage Yields for Methionyl-Serine and Methionyl-Threonine Peptide Bonds". Analytical Biochemistry. 266 (1): 1–8. doi:10.1006/abio.1998.2945. PMID 9887207.
- ^ a b c d Kumar, V. (2005). "Cyanogen Bromide (CNBr)" (PDF). Synlett. 2005 (10): 1638–1639. doi:10.1055/s-2005-869872. Art ID: V12705ST.
- ^ a b "Cyanogen Bromide HSDB 708". HSDB. NIH / NLM. 2009-04-07.
- ^ Lunn, G.; Sansone, E. B. (1985). "Destruction of Cyanogen Bromide and Inorganic Cyanides". Analytical Biochemistry. 147 (1): 245–250. doi:10.1016/0003-2697(85)90034-X. PMID 4025821.
Further reading
[edit]- Gross, E.; Witkop, B. (1962). "Nonenzymatic Cleavage of Peptide Bonds: The Methionine Residues in Bovine Pancreatic Ribonuclease" (PDF). Journal of Biological Chemistry. 237 (6): 1856–1860. doi:10.1016/S0021-9258(19)73948-9. PMID 13902203.
- Inglis, A. S.; Edman, P. (1970). "Mechanism of Cyanogen Bromide Reaction with Methionine in Peptides and Proteins". Analytical Biochemistry. 37 (1): 73–80. doi:10.1016/0003-2697(70)90259-9. PMID 5506566.
External links
[edit]- "Cyanogen Bromide MSDS Number: C6600". J. T. Baker. 1996-08-12.
- Teeri, A. E. (1948). "Thiamine and the Cyanogen Bromide Reaction". Journal of Biological Chemistry. 173 (2): 503–505. doi:10.1016/S0021-9258(18)57422-6. PMID 18910706.