Methyldiazonium: Difference between revisions
Appearance
Content deleted Content added
Omit counter-anion from structure |
added Category:Methyl compounds using HotCat |
||
| (5 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{Chembox |
{{Chembox |
||
<!-- Images --> |
<!-- Images -->| ImageFile = Methyldiazonium.png |
||
| ImageCaption = |
|||
| ImageFile = Methyldiazonium.png |
|||
| |
| OtherNames = |
||
| IUPACName = Methyldiazynium |
|||
|Section1={{Chembox Identifiers |
| Section1 = {{Chembox Identifiers |
||
| CASNo = 20404-06-2 |
| CASNo = 20404-06-2 |
||
| ChEBI = 176914 |
| ChEBI = 176914 |
||
| Line 15: | Line 16: | ||
| SMILES = C[N+]#N |
| SMILES = C[N+]#N |
||
}} |
}} |
||
|Section2={{Chembox Properties |
| Section2 = {{Chembox Properties |
||
|C=1|H=3|N=2|Formula_Charge=+ |
|C=1|H=3|N=2|Formula_Charge=+ |
||
|pKa = <10 |
|||
}} |
|||
| ⚫ | |||
|Section8={{Chembox Related |
|||
| ⚫ | |||
}} |
}} |
||
}} |
}} |
||
'''Methyldiazonium''' is an [[organic compound]] consisting of a [[methyl group]] attached to a [[diazo group]]. This [[cation]] |
'''Methyldiazonium''' is an [[organic compound]] consisting of a [[methyl group]] attached to a [[diazo group]]. This [[cation]] is the [[conjugate acid]] of [[diazomethane]], with an estimated [[pKa|p''K''<sub>a</sub>]]<10.<ref>{{Cite journal|last1=Fei|first1=Na|last2=Sauter|first2=Basilius|last3=Gillingham|first3=Dennis|date=2016|title=The p''K''<sub>a</sub> of Brønsted acids controls their reactivity with diazo compounds|journal=Chemical Communications|language=en|volume=52|issue=47|pages=7501–7504|doi=10.1039/C6CC03561B|pmid=27212133|doi-access=free}}</ref> |
||
:[[File:Methyldiazonium acidity equilibrium.png| |
:[[File:Methyldiazonium acidity equilibrium.png|250px]] |
||
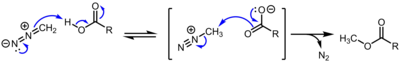
It is an [[chemical intermediate|intermediate]] in methylation reactions of diazomethane with acidic hydroxyl compounds, such as conversion of [[carboxylic acid]]s to [[methyl ester]]s and [[phenol]]s to [[methyl ether]]s.<ref>{{cite journal |title= Mechanism of Methyl Esterification of Carboxylic Acids by Trimethylsilyldiazomethane |first1= Erik |last1= Kühnel |first2= David D. P. |last2= Laffan |first3= Guy C. |last3= Lloyd-Jones |first4= Teresa |last4= Martínez del Campo |first5= Ian R. |last5= Shepperson |first6= Jennifer L. |last6= Slaughter |doi= 10.1002/anie.200702131 |journal= Angew. Chem. Int. Ed. Engl. |volume= 46 |issue= 37 |year= 2007 |pages= 7075–7078 |pmid= 17691089 }}</ref> |
It is an [[chemical intermediate|intermediate]] in methylation reactions of diazomethane with acidic hydroxyl compounds, such as conversion of [[carboxylic acid]]s to [[methyl ester]]s and [[phenol]]s to [[methyl ether]]s.<ref>{{cite journal |title= Mechanism of Methyl Esterification of Carboxylic Acids by Trimethylsilyldiazomethane |first1= Erik |last1= Kühnel |first2= David D. P. |last2= Laffan |first3= Guy C. |last3= Lloyd-Jones |first4= Teresa |last4= Martínez del Campo |first5= Ian R. |last5= Shepperson |first6= Jennifer L. |last6= Slaughter |doi= 10.1002/anie.200702131 |journal= Angew. Chem. Int. Ed. Engl. |volume= 46 |issue= 37 |year= 2007 |pages= 7075–7078 |pmid= 17691089 }}</ref> |
||
:[[File:Diazomethanemethylation.png|400px]] |
|||
It has been implicated as the [[metabolism|metabolite]] of [[N-Nitrosodimethylamine|''N''-nitrosodimethylamine]] responsible for the observed [[carcinogenicity]] of that compound.<ref name=Tricker>{{cite journal |pmid=2017213 |title= Carcinogenic ''N''-nitrosamines in the Diet: Occurrence, Formation, Mechanisms and Carcinogenic Potential |journal= Mutation Research/Genetic Toxicology |volume= 259 |issue= 3–4 |pages= 277–289 |year= 1991 |last1= Tricker |first1= A. R. |last2= Preussmann |first2= R. |doi= 10.1016/0165-1218(91)90123-4 }}</ref> |
|||
:[[File:Ndma activ.svg|400px]] |
|||
== References == |
== References == |
||
| Line 36: | Line 39: | ||
[[Category:Cations]] |
[[Category:Cations]] |
||
[[Category:Methylating agents]] |
[[Category:Methylating agents]] |
||
[[Category:Methyl compounds]] |
|||
Latest revision as of 08:55, 2 September 2024

| |
| Names | |
|---|---|
| IUPAC name
Methyldiazynium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH3N2+ | |
| Molar mass | 43.048 g·mol−1 |
| Acidity (pKa) | <10 |
| Conjugate base | Diazomethane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methyldiazonium is an organic compound consisting of a methyl group attached to a diazo group. This cation is the conjugate acid of diazomethane, with an estimated pKa<10.[1]
It is an intermediate in methylation reactions of diazomethane with acidic hydroxyl compounds, such as conversion of carboxylic acids to methyl esters and phenols to methyl ethers.[2]
It has been implicated as the metabolite of N-nitrosodimethylamine responsible for the observed carcinogenicity of that compound.[3]
References
[edit]- ^ Fei, Na; Sauter, Basilius; Gillingham, Dennis (2016). "The pKa of Brønsted acids controls their reactivity with diazo compounds". Chemical Communications. 52 (47): 7501–7504. doi:10.1039/C6CC03561B. PMID 27212133.
- ^ Kühnel, Erik; Laffan, David D. P.; Lloyd-Jones, Guy C.; Martínez del Campo, Teresa; Shepperson, Ian R.; Slaughter, Jennifer L. (2007). "Mechanism of Methyl Esterification of Carboxylic Acids by Trimethylsilyldiazomethane". Angew. Chem. Int. Ed. Engl. 46 (37): 7075–7078. doi:10.1002/anie.200702131. PMID 17691089.
- ^ Tricker, A. R.; Preussmann, R. (1991). "Carcinogenic N-nitrosamines in the Diet: Occurrence, Formation, Mechanisms and Carcinogenic Potential". Mutation Research/Genetic Toxicology. 259 (3–4): 277–289. doi:10.1016/0165-1218(91)90123-4. PMID 2017213.