Methyldiazonium: Difference between revisions
Appearance
Content deleted Content added
கி.மூர்த்தி (talk | contribs) No edit summary |
added Category:Methyl compounds using HotCat |
||
| Line 39: | Line 39: | ||
[[Category:Cations]] |
[[Category:Cations]] |
||
[[Category:Methylating agents]] |
[[Category:Methylating agents]] |
||
[[Category:Methyl compounds]] |
|||
Latest revision as of 08:55, 2 September 2024

| |
| Names | |
|---|---|
| IUPAC name
Methyldiazynium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CH3N2+ | |
| Molar mass | 43.048 g·mol−1 |
| Acidity (pKa) | <10 |
| Conjugate base | Diazomethane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
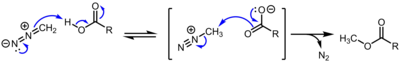
Methyldiazonium is an organic compound consisting of a methyl group attached to a diazo group. This cation is the conjugate acid of diazomethane, with an estimated pKa<10.[1]
It is an intermediate in methylation reactions of diazomethane with acidic hydroxyl compounds, such as conversion of carboxylic acids to methyl esters and phenols to methyl ethers.[2]
It has been implicated as the metabolite of N-nitrosodimethylamine responsible for the observed carcinogenicity of that compound.[3]
References
[edit]- ^ Fei, Na; Sauter, Basilius; Gillingham, Dennis (2016). "The pKa of Brønsted acids controls their reactivity with diazo compounds". Chemical Communications. 52 (47): 7501–7504. doi:10.1039/C6CC03561B. PMID 27212133.
- ^ Kühnel, Erik; Laffan, David D. P.; Lloyd-Jones, Guy C.; Martínez del Campo, Teresa; Shepperson, Ian R.; Slaughter, Jennifer L. (2007). "Mechanism of Methyl Esterification of Carboxylic Acids by Trimethylsilyldiazomethane". Angew. Chem. Int. Ed. Engl. 46 (37): 7075–7078. doi:10.1002/anie.200702131. PMID 17691089.
- ^ Tricker, A. R.; Preussmann, R. (1991). "Carcinogenic N-nitrosamines in the Diet: Occurrence, Formation, Mechanisms and Carcinogenic Potential". Mutation Research/Genetic Toxicology. 259 (3–4): 277–289. doi:10.1016/0165-1218(91)90123-4. PMID 2017213.