E-4 process: Difference between revisions
No edit summary |
m Clean up spacing around commas and other punctuation fixes, replaced: ,F → , F, ,T → , T |
||
| (20 intermediate revisions by 9 users not shown) | |||
| Line 1: | Line 1: | ||
{{More citations needed|date=April 2021}} |
|||
:''See also [[Ektachrome]] for full details of Kodak E-series processes.'' |
:''See also [[Ektachrome]] for full details of Kodak E-series processes.'' |
||
The '''E-4 process''' is a now outdated process for developing [[transparency (photography)|color reversal (transparency)]] photographic film |
The '''E-4 process''' is a now outdated process for developing [[transparency (photography)|color reversal (transparency)]] photographic film, which was introduced in 1966. |
||
==Drawbacks== |
|||
The process is infamous for two reasons: |
The process is infamous for two reasons: |
||
First, |
First, it uses the highly toxic [[boron hydride]]-based reversal agent [[tertiary butyl-amine borane]] (TBAB).{{efn|Not to be confused with [[tetra-n-butylammonium bromide]], which also is abbreviated as TBAB.}}<ref name=Jacobson80/>{{rp|379, Table LXVI}} Early releases of the consumer-sized version of the chemistry provided the TBAB in the form of a tablet, possibly to avoid the possibility of inhalation.<ref name=Talbert/> This was later changed to loose powder, likely as a countermeasure against inadvertent ingestion of the substance. |
||
Second, the |
Second, the prehardener agent contains [[formaldehyde]] and 2,5-dimethoxy[[tetrahydrofuran]],<ref name=Jacobson80/>{{rp|377, Formula 269}} which when mixed generates [[succinaldehyde]], a noxious gas which has been likened to tear gas.<ref name=Talbert/> Process E-6 films are hardened during manufacture, eliminating the prehardener step altogether and allowing them to be processed at {{cvt|100|F}}. |
||
==Steps== |
|||
The process is faster than E-3 and ran at 30°C (85°F, ± .5°F), about 6°C (10°F) higher than E-3. The ME-4 process was a motion picture variation of the E-4 process. |
|||
{{multiple image |align=right |direction=horizontal |title=Ektachrome film structure and exposure |
|||
|image1=E-6 step 00.svg |caption1=Structure |
|||
|image2=E-6 step 01.svg |caption2=Sample exposure to various colors}} |
|||
Ektachrome film has three separate light-sensitive layers; each layer is sensitive to a different group of wavelengths corresponding to red, green, and blue colors. When the film is exposed, each layer records a latent image based on its sensitivity. A yellow filter prevents blue light from exposing the green- and red-sensitive layers, which have some sensitivity to blue light.<ref name=Z119-1>{{cite web |url=http://www.kodak.com/global/plugins/acrobat/en/service/Zmanuals/z119-1.pdf |title=Process E-6 Using KODAK Chemicals, Process E-6 Publication Z-119 {{!}} Chapter 1: Processing solutions and their effects |publisher=[[Kodak]] |archive-url=https://web.archive.org/web/20050825145808/http://kodak.com/global/plugins/acrobat/en/service/Zmanuals/z119-1.pdf |archive-date=August 25, 2005 |url-status=dead}}</ref> |
|||
The E-4 process is faster than E-3; whereas E-3 required 15 steps and up to 70 minutes from start to finish,<ref name=Talbert>{{cite web |url=https://www.photomemorabilia.co.uk/Colour_Darkroom/Early_Kodak_Ektachrome.html |title=Kodak Ektachrome Colour Transparency films |author=Talbert, Michael |website=Photo Memorabilia |access-date=24 August 2023}}</ref><ref name=E-13>{{cite book |url=https://archive.org/details/kodakektachromef00east/ |title=Kodak Ektachrome Film, Publication No. E-13 |date=1955 |publisher=Eastman Kodak Company |url-access=registration}}</ref>{{rp|30–31}} E-4 was completed in approximately 50 minutes over 13 steps.<ref name=PS-1968/> E-4 runs at {{cvt|85|F}},<ref name=PS-1968>{{Cite magazine | url=https://books.google.com/books?id=kyYDAAAAMBAJ&pg=PA130 |title = Kodak's new E-4 kit: 50-Minute Cure for People Afraid to Develop Their Own Color Film |author=Wahl, Paul |magazine=Popular Science |date = April 1968 |pages=130–131}}</ref> about 10 °F (6 °C) higher than E-3. The temperature tolerance is ±1 °F for prehardener, ±{{frac|2}}°F for the first developer, and ±2–5 °F for all other steps.<ref name=PS-1968/> The ME-4 process was a motion picture variation of the E-4 process. |
|||
Process E-4 consisted of nine chemicals: |
|||
The major change for E-4 was the inclusion of a chemical reversal agent, which permits processing of the film without the manual re-exposure/fogging step required by the predecessor E-1 / E-2 / [[E-3 process]]es.<ref name=Talbert/><ref name=PS-1968/> |
|||
Prehardener, Neutralizer, First Developer, First Stop Bath, Color Developer, Second Stop Bath, Bleach, Fixer, Stabilizer |
|||
Total darkness |
Total darkness is required during the first four development steps; normal room light can be used for the remaining steps.<ref name=PS-1968/> |
||
{|class="wikitable" style="font-size:100%;text-align:left;" |
|||
| ⚫ | |||
|+E-4 Process<ref name=PS-1968/> |
|||
! colspan=3 | Step !! Schematic !! Time (min.) !! Temp. !! Description |
|||
|- |
|||
| rowspan=4 style="background:#000;" | |
|||
! 1 !! Prehardener |
|||
| rowspan=2 | |
|||
| 3 || {{cvt|85|F}} ±1 °F |
|||
| Tempers film for high-temperature processing |
|||
|- |
|||
! 2 !! Neutralizer |
|||
| 1 || {{cvt|83–87|F}} |
|||
| |
|||
|- |
|||
! 3 !! First developer |
|||
| rowspan=3 | [[File:E-6 step 02.svg|frameless|upright=0.7]] |
|||
| 7 || {{cvt|85|F}} ±{{frac|2}}°F |
|||
| Conventional black-and-white developer used to transform silver halide crystals exposed in all three layers as a negative image. |
|||
|- |
|||
! 4 !! First stop bath |
|||
| 2 || {{cvt|83–87|F}} |
|||
| Solution should not be reused for second stop bath (step 7) |
|||
|- |
|||
| rowspan=9 style="background:#fff;" | |
|||
! 5 !! Wash |
|||
| 4 || {{cvt|80–90|F}} |
|||
| Running water |
|||
|- |
|||
! 6 !! Color developer |
|||
| rowspan=3 | [[File:E-6 step 03.svg|frameless|upright=0.7]] |
|||
| 9 || {{cvt|83–87|F}} |
|||
| |
|||
|- |
|||
! 7 !! Second stop bath |
|||
| 3 || {{cvt|83–87|F}} |
|||
| Solution should not be reused from first stop bath (step 4) |
|||
|- |
|||
! 8 !! Wash |
|||
| 3 || {{cvt|80–90|F}} |
|||
| Running water |
|||
|- |
|||
! 9 !! Bleach |
|||
| rowspan=1 | [[File:E-6 step 04.svg|frameless|upright=0.7]] |
|||
| 5 || {{cvt|83–87|F}} |
|||
| Convert metallic silver to soluble particles |
|||
|- |
|||
! 10!! Fixer |
|||
| rowspan=4 | [[File:E-6 step 05.svg|frameless|upright=0.7]] |
|||
| 6 || {{cvt|83–87|F}} |
|||
| Dissolve silver particles, which can be recovered after processing |
|||
|- |
|||
! 11!! Wash |
|||
| 6 || {{cvt|80–90|F}} |
|||
| Running water |
|||
|- |
|||
! 12!! Stabilizer |
|||
| 1 || {{cvt|83–87|F}} |
|||
| |
|||
|- |
|||
! 13!! Dry |
|||
| var. || <{{cvt|110|F}} |
|||
| |
|||
|} |
|||
==History== |
|||
Today the process is discontinued but was used up until 1996 for Kodak IE Color Infrared film. This was due to legal commitment by Kodak to provide the process for 30 years. |
|||
[[File:Kodak Ektachrome IE 135-20 Infrared Slide Film.jpg|thumb|right|Kodak Ektachrome Infrared film using E-4 process]] |
|||
E-4 processed film is color stable for about 30 years.<ref>{{Cite web | url=https://www.digitalrev.com/article/ektachrome-a-look-back | title=Ektachrome: A Look Back| date=25 January 2017}}</ref> |
|||
| ⚫ | |||
The E-4 process has been discontinued since 1996; after 1976 it was used solely for Kodak IE color [[Infrared photography|infrared film]],<ref>{{cite magazine |url=https://books.google.com/books?id=_eMDAAAAMBAJ&pg=PA100 |title=Inner Visions |author=Ensanian, Armand |date=July 1988 |magazine=Popular Mechanics |pages=100–101 |access-date=24 August 2023 |quote=Color IR film has one drawback. It is not readily processed because it requires the old E-4 chemistry.}}</ref> due to a legal commitment by Kodak to provide process support for 30 years after introduction. Kodak discontinued E-4 processing in 1985, but independent photofinishers continued to support the process.<ref>{{cite magazine |url=https://books.google.com/books?id=LxmeZrp-lukC&pg=PA114 |title=Pop Photo Snapshots: Bad and good news from Kodak |author=Rothschild, Norman |date=December 1985 |magazine=Popular Photography |pages=28–32;114 |access-date=24 August 2023 |quote=Eastman Kodak no longer offers processing for E-4 films such as Ektachrome Infrared and Kodak Microphotography color-slide films. However, there are more than a dozen independent labs in the U.S. that offer this service.}}</ref> The E-4 chemicals were reverse-engineered and substitute formulae were published in the ''[[British Journal of Photography]] Annual'' in 1977.<ref name=Jacobson80>{{cite book |chapter-url=https://archive.org/details/DevelopingTheNegativeTechnique/page/n375/mode/2up |title=Developing: The Negative Technique |author1=Jacobson, Kurt I. |author2=Jacobson, Ralph Eric |date=1980 |edition=Eighteenth revised |publisher=Focal Press |location=London |isbn=0-240-44770-0 |access-date=24 August 2023 |chapter=Processing Colour Films |pages=363–383}}</ref>{{rp|374}} |
|||
==Notes== |
|||
{{notelist}} |
|||
== References == |
|||
{{Reflist}} |
|||
== External links == |
== External links == |
||
* [http://www.kodak.com/global/en/professional/support/techPubs/cis111/cis111.jhtml Kodak specifications for hand mixing of chemistry] |
* [http://www.kodak.com/global/en/professional/support/techPubs/cis111/cis111.jhtml Kodak specifications for hand mixing of chemistry] |
||
* {{cite web |url=https://www.mat.uc.pt/~rps/photos/FAQ_e4.txt |title=More than you want to know about E-4 |date=19 May 1995}} |
|||
===Processing of older Ektachrome films (including Process E-4)=== |
===Processing of older Ektachrome films (including Process E-4)=== |
||
* [http://www.processc22.co.uk Process C-22] UK and Europe |
|||
* [http://www.filmrescue.com Film Rescue] USA and Canada |
* [http://www.filmrescue.com Film Rescue] USA and Canada |
||
* [http://www.rockymountainfilm.com Rocky Mountain] USA |
* [http://www.rockymountainfilm.com Rocky Mountain] USA |
||
Latest revision as of 00:01, 7 September 2024
This article needs additional citations for verification. (April 2021) |
- See also Ektachrome for full details of Kodak E-series processes.
The E-4 process is a now outdated process for developing color reversal (transparency) photographic film, which was introduced in 1966.
Drawbacks
[edit]The process is infamous for two reasons:
First, it uses the highly toxic boron hydride-based reversal agent tertiary butyl-amine borane (TBAB).[a][1]: 379, Table LXVI Early releases of the consumer-sized version of the chemistry provided the TBAB in the form of a tablet, possibly to avoid the possibility of inhalation.[2] This was later changed to loose powder, likely as a countermeasure against inadvertent ingestion of the substance.
Second, the prehardener agent contains formaldehyde and 2,5-dimethoxytetrahydrofuran,[1]: 377, Formula 269 which when mixed generates succinaldehyde, a noxious gas which has been likened to tear gas.[2] Process E-6 films are hardened during manufacture, eliminating the prehardener step altogether and allowing them to be processed at 100 °F (38 °C).
Steps
[edit]Ektachrome film has three separate light-sensitive layers; each layer is sensitive to a different group of wavelengths corresponding to red, green, and blue colors. When the film is exposed, each layer records a latent image based on its sensitivity. A yellow filter prevents blue light from exposing the green- and red-sensitive layers, which have some sensitivity to blue light.[3]
The E-4 process is faster than E-3; whereas E-3 required 15 steps and up to 70 minutes from start to finish,[2][4]: 30–31 E-4 was completed in approximately 50 minutes over 13 steps.[5] E-4 runs at 85 °F (29 °C),[5] about 10 °F (6 °C) higher than E-3. The temperature tolerance is ±1 °F for prehardener, ±1⁄2°F for the first developer, and ±2–5 °F for all other steps.[5] The ME-4 process was a motion picture variation of the E-4 process.
The major change for E-4 was the inclusion of a chemical reversal agent, which permits processing of the film without the manual re-exposure/fogging step required by the predecessor E-1 / E-2 / E-3 processes.[2][5]
Total darkness is required during the first four development steps; normal room light can be used for the remaining steps.[5]
| Step | Schematic | Time (min.) | Temp. | Description | ||
|---|---|---|---|---|---|---|
| 1 | Prehardener | 3 | 85 °F (29 °C) ±1 °F | Tempers film for high-temperature processing | ||
| 2 | Neutralizer | 1 | 83–87 °F (28–31 °C) | |||
| 3 | First developer | 
|
7 | 85 °F (29 °C) ±1⁄2°F | Conventional black-and-white developer used to transform silver halide crystals exposed in all three layers as a negative image. | |
| 4 | First stop bath | 2 | 83–87 °F (28–31 °C) | Solution should not be reused for second stop bath (step 7) | ||
| 5 | Wash | 4 | 80–90 °F (27–32 °C) | Running water | ||
| 6 | Color developer | 
|
9 | 83–87 °F (28–31 °C) | ||
| 7 | Second stop bath | 3 | 83–87 °F (28–31 °C) | Solution should not be reused from first stop bath (step 4) | ||
| 8 | Wash | 3 | 80–90 °F (27–32 °C) | Running water | ||
| 9 | Bleach | 
|
5 | 83–87 °F (28–31 °C) | Convert metallic silver to soluble particles | |
| 10 | Fixer | 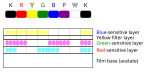
|
6 | 83–87 °F (28–31 °C) | Dissolve silver particles, which can be recovered after processing | |
| 11 | Wash | 6 | 80–90 °F (27–32 °C) | Running water | ||
| 12 | Stabilizer | 1 | 83–87 °F (28–31 °C) | |||
| 13 | Dry | var. | <110 °F (43 °C) | |||
History
[edit]
E-4 processed film is color stable for about 30 years.[6]
The process largely was phased out in 1976 with the introduction of the E-6 process, which is more environmentally friendly due to its lack of toxic chemicals. E-6 avoids the use of TBAB by adding a separate reversal bath containing the tin salt stannous chloride.
The E-4 process has been discontinued since 1996; after 1976 it was used solely for Kodak IE color infrared film,[7] due to a legal commitment by Kodak to provide process support for 30 years after introduction. Kodak discontinued E-4 processing in 1985, but independent photofinishers continued to support the process.[8] The E-4 chemicals were reverse-engineered and substitute formulae were published in the British Journal of Photography Annual in 1977.[1]: 374
Notes
[edit]- ^ Not to be confused with tetra-n-butylammonium bromide, which also is abbreviated as TBAB.
References
[edit]- ^ a b c Jacobson, Kurt I.; Jacobson, Ralph Eric (1980). "Processing Colour Films". Developing: The Negative Technique (Eighteenth revised ed.). London: Focal Press. pp. 363–383. ISBN 0-240-44770-0. Retrieved 24 August 2023.
- ^ a b c d Talbert, Michael. "Kodak Ektachrome Colour Transparency films". Photo Memorabilia. Retrieved 24 August 2023.
- ^ "Process E-6 Using KODAK Chemicals, Process E-6 Publication Z-119 | Chapter 1: Processing solutions and their effects" (PDF). Kodak. Archived from the original (PDF) on August 25, 2005.
- ^ Kodak Ektachrome Film, Publication No. E-13. Eastman Kodak Company. 1955.
- ^ a b c d e f Wahl, Paul (April 1968). "Kodak's new E-4 kit: 50-Minute Cure for People Afraid to Develop Their Own Color Film". Popular Science. pp. 130–131.
- ^ "Ektachrome: A Look Back". 25 January 2017.
- ^ Ensanian, Armand (July 1988). "Inner Visions". Popular Mechanics. pp. 100–101. Retrieved 24 August 2023.
Color IR film has one drawback. It is not readily processed because it requires the old E-4 chemistry.
- ^ Rothschild, Norman (December 1985). "Pop Photo Snapshots: Bad and good news from Kodak". Popular Photography. pp. 28–32, 114. Retrieved 24 August 2023.
Eastman Kodak no longer offers processing for E-4 films such as Ektachrome Infrared and Kodak Microphotography color-slide films. However, there are more than a dozen independent labs in the U.S. that offer this service.
External links
[edit]- Kodak specifications for hand mixing of chemistry
- "More than you want to know about E-4". 19 May 1995.
Processing of older Ektachrome films (including Process E-4)
[edit]- Film Rescue USA and Canada
- Rocky Mountain USA


