Tubulin alpha-1A chain: Difference between revisions
KolbertBot (talk | contribs) m Bot: HTTP→HTTPS |
Citation bot (talk | contribs) Altered issue. Formatted dashes. | Use this bot. Report bugs. | Suggested by Boghog | #UCB_webform |
||
| (24 intermediate revisions by 15 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Protein-coding gene in the species Homo sapiens}} |
|||
{{cs1 config|name-list-style=vanc|display-authors=6}} |
|||
{{Infobox_gene}} |
{{Infobox_gene}} |
||
'''Tubulin alpha-1A chain''' is a [[protein]] that in humans is encoded by the ''TUBA1A'' [[gene]].<ref name="pmid11504633">{{cite journal | vauthors = Crabtree DV, Ojima I, Geng X, Adler AJ | title = Tubulins in the primate retina: evidence that xanthophylls may be endogenous ligands for the paclitaxel-binding site | journal = Bioorganic & Medicinal Chemistry | volume = 9 | issue = 8 | pages = |
'''Tubulin alpha-1A chain''' is a [[protein]] that in humans is encoded by the ''TUBA1A'' [[gene]].<ref name="pmid11504633">{{cite journal | vauthors = Crabtree DV, Ojima I, Geng X, Adler AJ | title = Tubulins in the primate retina: evidence that xanthophylls may be endogenous ligands for the paclitaxel-binding site | journal = Bioorganic & Medicinal Chemistry | volume = 9 | issue = 8 | pages = 1967–1976 | date = August 2001 | pmid = 11504633 | doi = 10.1016/S0968-0896(01)00103-1 }}</ref><ref name="pmid3839072">{{cite journal | vauthors = Hall JL, Cowan NJ | title = Structural features and restricted expression of a human alpha-tubulin gene | journal = Nucleic Acids Research | volume = 13 | issue = 1 | pages = 207–223 | date = January 1985 | pmid = 3839072 | pmc = 340985 | doi = 10.1093/nar/13.1.207 }}</ref><ref name="entrez">{{cite web | title = Entrez Gene: TUBA1A tubulin, alpha 1a| url = https://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=ShowDetailView&TermToSearch=7846}}</ref> |
||
Tubulin alpha-1A chain is a type of alpha-tubulin involved in the formation of [[microtubule]]s, which are structural proteins that play a role in the cytoskeletal structure. Microtubules are composed of heterodimers of alpha- and beta-tubulin molecules. Tubulin alpha-1A (TUBA1A) is a primary alpha-tubulin expressed in the human fetal brain, specifically found in that structure.<ref name="PMC370035">{{cite journal | vauthors = Cowan NJ, Dobner PR, Fuchs EV, Cleveland DW | title = Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions | journal = Molecular and Cellular Biology | volume = 3 | issue = 10 | pages = 1738–1745 | date = October 1983 | pmid = 6646120 | pmc = 370035 | doi = 10.1128/mcb.3.10.1738 }}</ref> |
|||
==Background== |
|||
TUBA1A is a structural gene that encodes for Tubulin, Alpha 1A product. TUBA1A product is an alpha-tubulin that participates in the formation of microtubules - structural proteins that participate in cytoskeletal structure. Specifically, microtubules are composed of a heterodimer of alpha and beta-tubulin molecules. Cowan et al. demonstrated that bα1 is a primary α-tubulin of the human fetal brain, and that it is expressed solely in that structure, by way of Northern blot.<ref name="PMC370035">{{cite journal|last1=Cowan|first1=N. J.|last2=Dobner|first2=P. R.|last3=Fuchs|first3=E. V.|last4=Cleveland|first4=D. W.|title=Expression of Human α-Tubulin Genes: Interspecies Conversion of 3' Untranslated Regions|journal=Molecular and Cell Biology|date=1983|volume=3|issue=10|pages=1738–1739, 1742|pmid=6646120|accessdate=10 April 2017|pmc=370035}}</ref> Miller et al. further elaborated on the role of α-tubulins and the process of neuronal development and maturation, comparing the expressions of rat α-tubulins Tα1 and T26. These two rat α-tubulins are homologs of bα1 and kα1 showing that a rat homolog of human TUBA1A (Tα1) had elevated expression during the extension of neuronal processes. Culturing of pheochromocytoma cells with Nerve Growth Factor (NGF) induced differentiation and the development of neuronal processes. Northern blot assay showed markedly elevated levels of Tα1 mRNA expression; T26 mRNA expression increased minimally with exposure to NGF.<ref name="PMC2114727">{{cite journal|last1=Mill|first1=F. D.|last2=Naus|first2=C. C.|last3=Durand|first3=M.|last4=Bloom|first4=F. E.|last5=Milner|first5=R. J.|title=Isotypes of alpha-tubulin are differentially regulated during neuronal maturation|journal=The Journal of Cell Biology|date=1987|volume=105|issue=6|pages=3065–3073|pmid=3693406|accessdate=10 April 2017|pmc=2114727}}</ref> These data suggest that TUBA1A models the brain by participating in the directing of neuronal migration through the ability of microtubules to readily form and break polymers to extend and retract processes to induce nucleokinesis.<ref>{{cite journal|last1=Sakakaibara|first1=A.|last2=Ando|first2=R.|last3=Spair|first3=T.|last4=Tanaka|first4=T.|title=Microtubule dynamics in neuronal morphogenesis|journal=Open Biology|date=July 2013|volume=3|issue=7|pages=1–2|doi=10.1098/rsob.130061|pmid=23864552|accessdate=10 April 2017|pmc=3728923}}</ref> Poirier et al. used RNA in situ hybridization to show TUBA1A expression in mice embryo; embryo sections from embryonic day 16.5 “showed a strong labeling in the telencephalon, diencephalon, and mesencephalon, the developing cerebellum, the brainstem, the spinal cord, and the dorsal root ganglia”.<ref name="pmid17584854">{{cite journal|last1=Poirier|first1=K.|last2=Keays|first2=D. A.|last3=Francis|first3=F.|last4=Saillour|first4=Y.|last5=Bahi|first5=N.|last6=Manouvrier|first6=S.|last7=Fallet-Bianco|first7=C.|last8=Paquier|first8=L.|last9=Toutain|first9=A.|last10=Tuy|first10=F. P. D.|last11=Bienvenu|first11=T.|last12=Joriot|first12=S.|last13=Odent|first13=S.|last14=Ville|first14=D.|last15=Desguerre|first15=I.|last16=Goldenberg|first16=A.|last17=Moutard|first17=M.-L.|last18=Fryns|first18=J.-P.|last19=van Esch|first19=H.|last20=Harvey|first20=R. J.|last21=Siebold|first21=C.|last22=Flint|first22=J.|last23=Beldjord|first23=C.|last24=Chelly|first24=J.|title=Large Spectrum of Lissencephaly and Pachygyria Phenotypes Resulting from De Novo Missense Mutations in Tubulin Alpha 1A (TUBA1A)|journal=Human Mutation|date=November 2007|volume=28|issue=11|pages=1058–1061|doi=10.1002/humu.20572|pmid=17584854|accessdate=10 April 2017}}</ref> |
|||
== Function == |
== Function == |
||
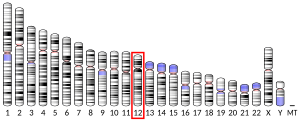
Microtubules of the eukaryotic cytoskeleton perform essential and diverse functions and are composed of a heterodimer of alpha and beta tubulins. The genes encoding these microtubule constituents belong to the tubulin superfamily, which is composed of six distinct families. Genes from the alpha, beta and gamma tubulin families are found in all eukaryotes. The alpha and beta tubulins represent the major components of microtubules, while gamma tubulin plays a critical role in the nucleation of microtubule assembly. There are multiple alpha and beta tubulin genes, which are highly conserved among species. This gene encodes alpha tubulin and is highly similar to mouse and rat Tuba1 gene. [[Northern blot]]ting studies have shown that the gene expression is predominantly found in morphologically differentiated neurologic cells. This gene is one of three alpha-tubulin genes in a cluster on chromosome 12q.<ref name="entrez" /> |
Microtubules of the eukaryotic cytoskeleton perform essential and diverse functions and are composed of a heterodimer of alpha and beta tubulins. The genes encoding these microtubule constituents belong to the tubulin superfamily, which is composed of six distinct families. Genes from the alpha, beta and gamma tubulin families are found in all eukaryotes. The alpha and beta tubulins represent the major components of microtubules, while gamma tubulin plays a critical role in the nucleation of microtubule assembly. There are multiple alpha and beta tubulin genes, which are highly conserved among species. This gene encodes alpha tubulin and is highly similar to mouse and rat Tuba1 gene. [[Northern blot]]ting studies have shown that the gene expression is predominantly found in morphologically differentiated neurologic cells. This gene is one of three alpha-tubulin genes in a cluster on chromosome 12q.<ref name="entrez" /> |
||
Alpha-tubulins, including TUBA1A, are involved in neuronal development and maturation. Studies have shown that the rat homologs of human TUBA1A, such as Tα1, exhibit elevated expression during the extension of neuronal processes. In experiments where pheochromocytoma cells were cultured with Nerve Growth Factor (NGF), differentiation and the development of neuronal processes were observed, accompanied by a significant increase in Tα1 mRNA expression, while T26 mRNA expression showed minimal change.<ref name="PMC2114727">{{cite journal | vauthors = Miller FD, Naus CC, Durand M, Bloom FE, Milner RJ | title = Isotypes of alpha-tubulin are differentially regulated during neuronal maturation | journal = The Journal of Cell Biology | volume = 105 | issue = 6 Pt 2 | pages = 3065–3073 | date = December 1987 | pmid = 3693406 | pmc = 2114727 | doi = 10.1083/jcb.105.6.3065 }}</ref> |
|||
TUBA1A is believed to play a role in neuronal migration by regulating microtubule dynamics, enabling the rapid formation and disassembly of polymers, which allows for the extension and retraction of processes necessary for nucleokinesis.<ref>{{cite journal | vauthors = Sakakibara A, Ando R, Sapir T, Tanaka T | title = Microtubule dynamics in neuronal morphogenesis | journal = Open Biology | volume = 3 | issue = 7 | pages = 130061 | date = July 2013 | pmid = 23864552 | pmc = 3728923 | doi = 10.1098/rsob.130061 }}</ref> |
|||
RNA in situ hybridization studies demonstrated the expression of TUBA1A in mouse embryos. Embryonic day 16.5 sections showed strong labeling in the telencephalon, diencephalon, mesencephalon, developing cerebellum, brainstem, spinal cord, and dorsal root ganglia.<ref name="pmid17584854">{{cite journal | vauthors = Poirier K, Keays DA, Francis F, Saillour Y, Bahi N, Manouvrier S, Fallet-Bianco C, Pasquier L, Toutain A, Tuy FP, Bienvenu T, Joriot S, Odent S, Ville D, Desguerre I, Goldenberg A, Moutard ML, Fryns JP, van Esch H, Harvey RJ, Siebold C, Flint J, Beldjord C, Chelly J | title = Large spectrum of lissencephaly and pachygyria phenotypes resulting from de novo missense mutations in tubulin alpha 1A (TUBA1A) | journal = Human Mutation | volume = 28 | issue = 11 | pages = 1055–1064 | date = November 2007 | pmid = 17584854 | doi = 10.1002/humu.20572 | s2cid = 22681290 | doi-access = free }}</ref> |
|||
== Interactions == |
== Interactions == |
||
TUBA1A has been shown to [[Protein-protein interaction|interact]] with [[PAFAH1B1]].<ref name=pmid9384577>{{cite journal | vauthors = Sapir T, Elbaum M, Reiner O | title = Reduction of microtubule catastrophe events by LIS1, platelet-activating factor acetylhydrolase subunit | journal = The EMBO Journal | volume = 16 | issue = 23 | pages = |
TUBA1A has been shown to [[Protein-protein interaction|interact]] with [[PAFAH1B1]].<ref name=pmid9384577>{{cite journal | vauthors = Sapir T, Elbaum M, Reiner O | title = Reduction of microtubule catastrophe events by LIS1, platelet-activating factor acetylhydrolase subunit | journal = The EMBO Journal | volume = 16 | issue = 23 | pages = 6977–6984 | date = December 1997 | pmid = 9384577 | pmc = 1170301 | doi = 10.1093/emboj/16.23.6977 }}</ref> |
||
== |
== Animal models == |
||
| ⚫ | Keays et al. describe a mouse with a mutation of the TUBA1A gene induced by N-ethyl-N-nitrosourea. The relevant point mutation resulted in S140G;<ref name="pmid17218254">{{cite journal | vauthors = Keays DA, Tian G, Poirier K, Huang GJ, Siebold C, Cleak J, Oliver PL, Fray M, Harvey RJ, Molnár Z, Piñon MC, Dear N, Valdar W, Brown SD, Davies KE, Rawlins JN, Cowan NJ, Nolan P, Chelly J, Flint J | title = Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans | journal = Cell | volume = 128 | issue = 1 | pages = 45–57 | date = January 2007 | pmid = 17218254 | pmc = 1885944 | doi = 10.1016/j.cell.2006.12.017 }}</ref> the site of the mutation participates in the N-site of the formed α-tubulin, and participates in stabilizing the α-β tubulin polymer by binding GTP at this site.<ref>{{cite journal | vauthors = Löwe J, Li H, Downing KH, Nogales E | title = Refined structure of alpha beta-tubulin at 3.5 A resolution | journal = Journal of Molecular Biology | volume = 313 | issue = 5 | pages = 1045–1057 | date = November 2001 | pmid = 11700061 | doi = 10.1006/jmbi.2001.5077 }}</ref> The S140G mutation resulted in the formation of a “compromised GTP binding pocket”. Authors note defects associated with cortical layers II/III and IV, especially in cortical neuronal migration (with respect to wild-type counterparts), showing that the S140G mutation has value as a model for detailing disease associated with the Human TUBA homolog.<ref name="pmid17218254" /> |
||
| ⚫ | Mutations to the TUBA1A gene manifest clinically as Type 3 Lissencephaly. In general, lissencephaly is characterized by agyria (lacking of gyri and sulci to the brain – a smooth brain), seizure activity, failure to thrive, as well as intellectual disability and psychomotor retardation, often to a profound degree.<ref name="pmid17584854"/> |
||
| ⚫ | The symptoms of Lis3 Lissencephaly are not especially different from generalized lissencephaly (Lis1, related to PAFAH1B1). Diagnosis of lissencephaly generally is made from the symptom profile, while attribution to a specific type is obtained by microarray. Treatment is symptomatic; anti-convulsive drugs for seizure activity, g-button gastrostomy to feed the child, physical therapy for muscle disorders. |
||
== Clinical significance == |
|||
==Animal Model== |
|||
| ⚫ | Mutations to the TUBA1A gene manifest clinically as Type 3 Lissencephaly. In general, lissencephaly is characterized by agyria (lacking of gyri and sulci to the brain – a smooth brain), seizure activity, failure to thrive, as well as intellectual disability and psychomotor retardation, often to a profound degree.<ref name="pmid17584854"/> |
||
| ⚫ | Keays et al. describe a mouse with a mutation of the TUBA1A gene induced by N-ethyl-N-nitrosourea. The relevant point mutation resulted in S140G;<ref name="pmid17218254">{{cite journal| |
||
| ⚫ | The symptoms of Lis3 Lissencephaly are not especially different from generalized lissencephaly (Lis1, related to PAFAH1B1). Diagnosis of lissencephaly generally is made from the symptom profile, while attribution to a specific type is obtained by microarray. Treatment is symptomatic; anti-convulsive drugs for seizure activity, g-button gastrostomy to feed the child, physical therapy for muscle disorders. TUBA1A mutation is common in [[microlissencephaly]] |
||
== References == |
== References == |
||
| Line 27: | Line 34: | ||
== Further reading == |
== Further reading == |
||
{{refbegin | 2}} |
{{refbegin | 2}} |
||
* {{cite journal | vauthors = Desai A, Mitchison TJ | title = Tubulin and FtsZ structures: functional and therapeutic implications | journal = BioEssays | volume = 20 | issue = 7 | pages = |
* {{cite journal | vauthors = Desai A, Mitchison TJ | title = Tubulin and FtsZ structures: functional and therapeutic implications | journal = BioEssays | volume = 20 | issue = 7 | pages = 523–527 | date = July 1998 | pmid = 9722999 | doi = 10.1002/(SICI)1521-1878(199807)20:7<523::AID-BIES1>3.0.CO;2-L }} |
||
* {{cite journal | vauthors = Oakley BR | title = An abundance of tubulins | journal = Trends in Cell Biology | volume = 10 | issue = 12 | pages = |
* {{cite journal | vauthors = Oakley BR | title = An abundance of tubulins | journal = Trends in Cell Biology | volume = 10 | issue = 12 | pages = 537–542 | date = December 2000 | pmid = 11121746 | doi = 10.1016/S0962-8924(00)01857-2 }} |
||
* {{cite journal | vauthors = Dutcher SK | title = The tubulin fraternity: alpha to eta | journal = Current Opinion in Cell Biology | volume = 13 | issue = 1 | pages = 49–54 | date = February 2001 | pmid = 11163133 | doi = 10.1016/S0955-0674(00)00173-3 }} |
* {{cite journal | vauthors = Dutcher SK | title = The tubulin fraternity: alpha to eta | journal = Current Opinion in Cell Biology | volume = 13 | issue = 1 | pages = 49–54 | date = February 2001 | pmid = 11163133 | doi = 10.1016/S0955-0674(00)00173-3 }} |
||
* {{cite journal | vauthors = Miller FD, Naus CC, Durand M, Bloom FE, Milner RJ | title = Isotypes of alpha-tubulin are differentially regulated during neuronal maturation | journal = The Journal of Cell Biology | volume = 105 | issue = 6 Pt 2 | pages = |
* {{cite journal | vauthors = Miller FD, Naus CC, Durand M, Bloom FE, Milner RJ | title = Isotypes of alpha-tubulin are differentially regulated during neuronal maturation | journal = The Journal of Cell Biology | volume = 105 | issue = 6 Pt 2 | pages = 3065–3073 | date = December 1987 | pmid = 3693406 | pmc = 2114727 | doi = 10.1083/jcb.105.6.3065 }} |
||
* {{cite journal | vauthors = Cowan NJ, Dobner PR, Fuchs EV, Cleveland DW | title = Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions | journal = Molecular and Cellular Biology | volume = 3 | issue = 10 | pages = |
* {{cite journal | vauthors = Cowan NJ, Dobner PR, Fuchs EV, Cleveland DW | title = Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions | journal = Molecular and Cellular Biology | volume = 3 | issue = 10 | pages = 1738–1745 | date = October 1983 | pmid = 6646120 | pmc = 370035 | doi = 10.1128/mcb.3.10.1738 }} |
||
* {{cite journal | vauthors = Alexandrova N, Niklinski J, Bliskovsky V, Otterson GA, Blake M, Kaye FJ, Zajac-Kaye M | title = The N-terminal domain of c-Myc associates with alpha-tubulin and microtubules in vivo and in vitro | journal = Molecular and Cellular Biology | volume = 15 | issue = 9 | pages = |
* {{cite journal | vauthors = Alexandrova N, Niklinski J, Bliskovsky V, Otterson GA, Blake M, Kaye FJ, Zajac-Kaye M | title = The N-terminal domain of c-Myc associates with alpha-tubulin and microtubules in vivo and in vitro | journal = Molecular and Cellular Biology | volume = 15 | issue = 9 | pages = 5188–5195 | date = September 1995 | pmid = 7651436 | pmc = 230766 | doi = 10.1128/MCB.15.9.5188 }} |
||
* {{cite journal | vauthors = Sapir T, Elbaum M, Reiner O | title = Reduction of microtubule catastrophe events by LIS1, platelet-activating factor acetylhydrolase subunit | journal = The EMBO Journal | volume = 16 | issue = 23 | pages = |
* {{cite journal | vauthors = Sapir T, Elbaum M, Reiner O | title = Reduction of microtubule catastrophe events by LIS1, platelet-activating factor acetylhydrolase subunit | journal = The EMBO Journal | volume = 16 | issue = 23 | pages = 6977–6984 | date = December 1997 | pmid = 9384577 | pmc = 1170301 | doi = 10.1093/emboj/16.23.6977 }} |
||
* {{cite journal | vauthors = Kinnunen T, Kaksonen M, Saarinen J, Kalkkinen N, Peng HB, Rauvala H | title = Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth | journal = The Journal of Biological Chemistry | volume = 273 | issue = 17 | pages = |
* {{cite journal | vauthors = Kinnunen T, Kaksonen M, Saarinen J, Kalkkinen N, Peng HB, Rauvala H | title = Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth | journal = The Journal of Biological Chemistry | volume = 273 | issue = 17 | pages = 10702–10708 | date = April 1998 | pmid = 9553134 | doi = 10.1074/jbc.273.17.10702 | doi-access = free }} |
||
* {{cite journal | vauthors = Faruki S, Geahlen RL, Asai DJ | title = Syk-dependent phosphorylation of microtubules in activated B-lymphocytes | journal = Journal of Cell Science | volume = 113 |
* {{cite journal | vauthors = Faruki S, Geahlen RL, Asai DJ | title = Syk-dependent phosphorylation of microtubules in activated B-lymphocytes | journal = Journal of Cell Science | volume = 113 | issue = 14 | pages = 2557–2565 | date = July 2000 | pmid = 10862713 | doi = 10.1242/jcs.113.14.2557 }} |
||
* {{cite journal | vauthors = Watts NR, Sackett DL, Ward RD, Miller MW, Wingfield PT, Stahl SS, Steven AC | title = HIV-1 rev depolymerizes microtubules to form stable bilayered rings | journal = The Journal of Cell Biology | volume = 150 | issue = 2 | pages = |
* {{cite journal | vauthors = Watts NR, Sackett DL, Ward RD, Miller MW, Wingfield PT, Stahl SS, Steven AC | title = HIV-1 rev depolymerizes microtubules to form stable bilayered rings | journal = The Journal of Cell Biology | volume = 150 | issue = 2 | pages = 349–360 | date = July 2000 | pmid = 10908577 | pmc = 2180222 | doi = 10.1083/jcb.150.2.349 }} |
||
* {{cite journal | vauthors = Germani A, Bruzzoni-Giovanelli H, Fellous A, Gisselbrecht S, Varin-Blank N, Calvo F | title = SIAH-1 interacts with alpha-tubulin and degrades the kinesin Kid by the proteasome pathway during mitosis | journal = Oncogene | volume = 19 | issue = 52 | pages = 5997–6006 | date = December 2000 | pmid = 11146551 | doi = 10.1038/sj.onc.1204002 }} |
* {{cite journal | vauthors = Germani A, Bruzzoni-Giovanelli H, Fellous A, Gisselbrecht S, Varin-Blank N, Calvo F | title = SIAH-1 interacts with alpha-tubulin and degrades the kinesin Kid by the proteasome pathway during mitosis | journal = Oncogene | volume = 19 | issue = 52 | pages = 5997–6006 | date = December 2000 | pmid = 11146551 | doi = 10.1038/sj.onc.1204002 | s2cid = 41279377 | doi-access = }} |
||
* {{cite journal | vauthors = Payton JE, Perrin RJ, Clayton DF, George JM | title = Protein-protein interactions of alpha-synuclein in brain homogenates and transfected cells | journal = Brain Research. Molecular Brain Research | volume = 95 | issue = |
* {{cite journal | vauthors = Payton JE, Perrin RJ, Clayton DF, George JM | title = Protein-protein interactions of alpha-synuclein in brain homogenates and transfected cells | journal = Brain Research. Molecular Brain Research | volume = 95 | issue = 1–2 | pages = 138–145 | date = November 2001 | pmid = 11687285 | doi = 10.1016/S0169-328X(01)00257-1 }} |
||
* {{cite journal | vauthors = Bifulco M, Laezza C, Stingo S, Wolff J | title = 2',3'-Cyclic nucleotide 3'-phosphodiesterase: a membrane-bound, microtubule-associated protein and membrane anchor for tubulin | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 99 | issue = 4 | pages = |
* {{cite journal | vauthors = Bifulco M, Laezza C, Stingo S, Wolff J | title = 2',3'-Cyclic nucleotide 3'-phosphodiesterase: a membrane-bound, microtubule-associated protein and membrane anchor for tubulin | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 99 | issue = 4 | pages = 1807–1812 | date = February 2002 | pmid = 11842207 | pmc = 122275 | doi = 10.1073/pnas.042678799 | doi-access = free | bibcode = 2002PNAS...99.1807B }} |
||
* {{cite journal | vauthors = Saugstad JA, Yang S, Pohl J, Hall RA, Conn PJ | title = Interaction between metabotropic glutamate receptor 7 and alpha tubulin | journal = Journal of Neurochemistry | volume = 80 | issue = 6 | pages = |
* {{cite journal | vauthors = Saugstad JA, Yang S, Pohl J, Hall RA, Conn PJ | title = Interaction between metabotropic glutamate receptor 7 and alpha tubulin | journal = Journal of Neurochemistry | volume = 80 | issue = 6 | pages = 980–988 | date = March 2002 | pmid = 11953448 | pmc = 2925652 | doi = 10.1046/j.0022-3042.2002.00778.x }} |
||
* {{cite journal | vauthors = Ivings L, Pennington SR, Jenkins R, Weiss JL, Burgoyne RD | title = Identification of Ca2+-dependent binding partners for the neuronal calcium sensor protein neurocalcin delta: interaction with actin, clathrin and tubulin | journal = The Biochemical Journal | volume = 363 | issue = Pt 3 | pages = 599–608 | date = May 2002 | pmid = 11964161 | pmc = 1222513 | doi = 10.1042/0264-6021:3630599 }} |
* {{cite journal | vauthors = Ivings L, Pennington SR, Jenkins R, Weiss JL, Burgoyne RD | title = Identification of Ca2+-dependent binding partners for the neuronal calcium sensor protein neurocalcin delta: interaction with actin, clathrin and tubulin | journal = The Biochemical Journal | volume = 363 | issue = Pt 3 | pages = 599–608 | date = May 2002 | pmid = 11964161 | pmc = 1222513 | doi = 10.1042/0264-6021:3630599 }} |
||
{{refend}} |
{{refend}} |
||
| ⚫ | |||
== External links == |
|||
* {{PDBe-KB2|Q71U36|Human Tubulin alpha-1A chain}} |
|||
* {{PDBe-KB2|P68369|Mouse Tubulin alpha-1A chain}} |
|||
| ⚫ | |||
Latest revision as of 06:11, 29 September 2024
| TUBA1A | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | TUBA1A, B-ALPHA-1, LIS3, TUBA3, tubulin alpha 1a | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 602529; MGI: 98869; HomoloGene: 68498; GeneCards: TUBA1A; OMA:TUBA1A - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Tubulin alpha-1A chain is a protein that in humans is encoded by the TUBA1A gene.[5][6][7]
Tubulin alpha-1A chain is a type of alpha-tubulin involved in the formation of microtubules, which are structural proteins that play a role in the cytoskeletal structure. Microtubules are composed of heterodimers of alpha- and beta-tubulin molecules. Tubulin alpha-1A (TUBA1A) is a primary alpha-tubulin expressed in the human fetal brain, specifically found in that structure.[8]
Function
[edit]Microtubules of the eukaryotic cytoskeleton perform essential and diverse functions and are composed of a heterodimer of alpha and beta tubulins. The genes encoding these microtubule constituents belong to the tubulin superfamily, which is composed of six distinct families. Genes from the alpha, beta and gamma tubulin families are found in all eukaryotes. The alpha and beta tubulins represent the major components of microtubules, while gamma tubulin plays a critical role in the nucleation of microtubule assembly. There are multiple alpha and beta tubulin genes, which are highly conserved among species. This gene encodes alpha tubulin and is highly similar to mouse and rat Tuba1 gene. Northern blotting studies have shown that the gene expression is predominantly found in morphologically differentiated neurologic cells. This gene is one of three alpha-tubulin genes in a cluster on chromosome 12q.[7]
Alpha-tubulins, including TUBA1A, are involved in neuronal development and maturation. Studies have shown that the rat homologs of human TUBA1A, such as Tα1, exhibit elevated expression during the extension of neuronal processes. In experiments where pheochromocytoma cells were cultured with Nerve Growth Factor (NGF), differentiation and the development of neuronal processes were observed, accompanied by a significant increase in Tα1 mRNA expression, while T26 mRNA expression showed minimal change.[9]
TUBA1A is believed to play a role in neuronal migration by regulating microtubule dynamics, enabling the rapid formation and disassembly of polymers, which allows for the extension and retraction of processes necessary for nucleokinesis.[10]
RNA in situ hybridization studies demonstrated the expression of TUBA1A in mouse embryos. Embryonic day 16.5 sections showed strong labeling in the telencephalon, diencephalon, mesencephalon, developing cerebellum, brainstem, spinal cord, and dorsal root ganglia.[11]
Interactions
[edit]TUBA1A has been shown to interact with PAFAH1B1.[12]
Animal models
[edit]Keays et al. describe a mouse with a mutation of the TUBA1A gene induced by N-ethyl-N-nitrosourea. The relevant point mutation resulted in S140G;[13] the site of the mutation participates in the N-site of the formed α-tubulin, and participates in stabilizing the α-β tubulin polymer by binding GTP at this site.[14] The S140G mutation resulted in the formation of a “compromised GTP binding pocket”. Authors note defects associated with cortical layers II/III and IV, especially in cortical neuronal migration (with respect to wild-type counterparts), showing that the S140G mutation has value as a model for detailing disease associated with the Human TUBA homolog.[13]
Clinical significance
[edit]Mutations to the TUBA1A gene manifest clinically as Type 3 Lissencephaly. In general, lissencephaly is characterized by agyria (lacking of gyri and sulci to the brain – a smooth brain), seizure activity, failure to thrive, as well as intellectual disability and psychomotor retardation, often to a profound degree.[11] The symptoms of Lis3 Lissencephaly are not especially different from generalized lissencephaly (Lis1, related to PAFAH1B1). Diagnosis of lissencephaly generally is made from the symptom profile, while attribution to a specific type is obtained by microarray. Treatment is symptomatic; anti-convulsive drugs for seizure activity, g-button gastrostomy to feed the child, physical therapy for muscle disorders. TUBA1A mutation is common in microlissencephaly
References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000167552 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000072235 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Crabtree DV, Ojima I, Geng X, Adler AJ (August 2001). "Tubulins in the primate retina: evidence that xanthophylls may be endogenous ligands for the paclitaxel-binding site". Bioorganic & Medicinal Chemistry. 9 (8): 1967–1976. doi:10.1016/S0968-0896(01)00103-1. PMID 11504633.
- ^ Hall JL, Cowan NJ (January 1985). "Structural features and restricted expression of a human alpha-tubulin gene". Nucleic Acids Research. 13 (1): 207–223. doi:10.1093/nar/13.1.207. PMC 340985. PMID 3839072.
- ^ a b "Entrez Gene: TUBA1A tubulin, alpha 1a".
- ^ Cowan NJ, Dobner PR, Fuchs EV, Cleveland DW (October 1983). "Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions". Molecular and Cellular Biology. 3 (10): 1738–1745. doi:10.1128/mcb.3.10.1738. PMC 370035. PMID 6646120.
- ^ Miller FD, Naus CC, Durand M, Bloom FE, Milner RJ (December 1987). "Isotypes of alpha-tubulin are differentially regulated during neuronal maturation". The Journal of Cell Biology. 105 (6 Pt 2): 3065–3073. doi:10.1083/jcb.105.6.3065. PMC 2114727. PMID 3693406.
- ^ Sakakibara A, Ando R, Sapir T, Tanaka T (July 2013). "Microtubule dynamics in neuronal morphogenesis". Open Biology. 3 (7): 130061. doi:10.1098/rsob.130061. PMC 3728923. PMID 23864552.
- ^ a b Poirier K, Keays DA, Francis F, Saillour Y, Bahi N, Manouvrier S, et al. (November 2007). "Large spectrum of lissencephaly and pachygyria phenotypes resulting from de novo missense mutations in tubulin alpha 1A (TUBA1A)". Human Mutation. 28 (11): 1055–1064. doi:10.1002/humu.20572. PMID 17584854. S2CID 22681290.
- ^ Sapir T, Elbaum M, Reiner O (December 1997). "Reduction of microtubule catastrophe events by LIS1, platelet-activating factor acetylhydrolase subunit". The EMBO Journal. 16 (23): 6977–6984. doi:10.1093/emboj/16.23.6977. PMC 1170301. PMID 9384577.
- ^ a b Keays DA, Tian G, Poirier K, Huang GJ, Siebold C, Cleak J, et al. (January 2007). "Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans". Cell. 128 (1): 45–57. doi:10.1016/j.cell.2006.12.017. PMC 1885944. PMID 17218254.
- ^ Löwe J, Li H, Downing KH, Nogales E (November 2001). "Refined structure of alpha beta-tubulin at 3.5 A resolution". Journal of Molecular Biology. 313 (5): 1045–1057. doi:10.1006/jmbi.2001.5077. PMID 11700061.
Further reading
[edit]- Desai A, Mitchison TJ (July 1998). "Tubulin and FtsZ structures: functional and therapeutic implications". BioEssays. 20 (7): 523–527. doi:10.1002/(SICI)1521-1878(199807)20:7<523::AID-BIES1>3.0.CO;2-L. PMID 9722999.
- Oakley BR (December 2000). "An abundance of tubulins". Trends in Cell Biology. 10 (12): 537–542. doi:10.1016/S0962-8924(00)01857-2. PMID 11121746.
- Dutcher SK (February 2001). "The tubulin fraternity: alpha to eta". Current Opinion in Cell Biology. 13 (1): 49–54. doi:10.1016/S0955-0674(00)00173-3. PMID 11163133.
- Miller FD, Naus CC, Durand M, Bloom FE, Milner RJ (December 1987). "Isotypes of alpha-tubulin are differentially regulated during neuronal maturation". The Journal of Cell Biology. 105 (6 Pt 2): 3065–3073. doi:10.1083/jcb.105.6.3065. PMC 2114727. PMID 3693406.
- Cowan NJ, Dobner PR, Fuchs EV, Cleveland DW (October 1983). "Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions". Molecular and Cellular Biology. 3 (10): 1738–1745. doi:10.1128/mcb.3.10.1738. PMC 370035. PMID 6646120.
- Alexandrova N, Niklinski J, Bliskovsky V, Otterson GA, Blake M, Kaye FJ, et al. (September 1995). "The N-terminal domain of c-Myc associates with alpha-tubulin and microtubules in vivo and in vitro". Molecular and Cellular Biology. 15 (9): 5188–5195. doi:10.1128/MCB.15.9.5188. PMC 230766. PMID 7651436.
- Sapir T, Elbaum M, Reiner O (December 1997). "Reduction of microtubule catastrophe events by LIS1, platelet-activating factor acetylhydrolase subunit". The EMBO Journal. 16 (23): 6977–6984. doi:10.1093/emboj/16.23.6977. PMC 1170301. PMID 9384577.
- Kinnunen T, Kaksonen M, Saarinen J, Kalkkinen N, Peng HB, Rauvala H (April 1998). "Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth". The Journal of Biological Chemistry. 273 (17): 10702–10708. doi:10.1074/jbc.273.17.10702. PMID 9553134.
- Faruki S, Geahlen RL, Asai DJ (July 2000). "Syk-dependent phosphorylation of microtubules in activated B-lymphocytes". Journal of Cell Science. 113 (14): 2557–2565. doi:10.1242/jcs.113.14.2557. PMID 10862713.
- Watts NR, Sackett DL, Ward RD, Miller MW, Wingfield PT, Stahl SS, et al. (July 2000). "HIV-1 rev depolymerizes microtubules to form stable bilayered rings". The Journal of Cell Biology. 150 (2): 349–360. doi:10.1083/jcb.150.2.349. PMC 2180222. PMID 10908577.
- Germani A, Bruzzoni-Giovanelli H, Fellous A, Gisselbrecht S, Varin-Blank N, Calvo F (December 2000). "SIAH-1 interacts with alpha-tubulin and degrades the kinesin Kid by the proteasome pathway during mitosis". Oncogene. 19 (52): 5997–6006. doi:10.1038/sj.onc.1204002. PMID 11146551. S2CID 41279377.
- Payton JE, Perrin RJ, Clayton DF, George JM (November 2001). "Protein-protein interactions of alpha-synuclein in brain homogenates and transfected cells". Brain Research. Molecular Brain Research. 95 (1–2): 138–145. doi:10.1016/S0169-328X(01)00257-1. PMID 11687285.
- Bifulco M, Laezza C, Stingo S, Wolff J (February 2002). "2',3'-Cyclic nucleotide 3'-phosphodiesterase: a membrane-bound, microtubule-associated protein and membrane anchor for tubulin". Proceedings of the National Academy of Sciences of the United States of America. 99 (4): 1807–1812. Bibcode:2002PNAS...99.1807B. doi:10.1073/pnas.042678799. PMC 122275. PMID 11842207.
- Saugstad JA, Yang S, Pohl J, Hall RA, Conn PJ (March 2002). "Interaction between metabotropic glutamate receptor 7 and alpha tubulin". Journal of Neurochemistry. 80 (6): 980–988. doi:10.1046/j.0022-3042.2002.00778.x. PMC 2925652. PMID 11953448.
- Ivings L, Pennington SR, Jenkins R, Weiss JL, Burgoyne RD (May 2002). "Identification of Ca2+-dependent binding partners for the neuronal calcium sensor protein neurocalcin delta: interaction with actin, clathrin and tubulin". The Biochemical Journal. 363 (Pt 3): 599–608. doi:10.1042/0264-6021:3630599. PMC 1222513. PMID 11964161.