Thionin: Difference between revisions
m Repair duplicate template args |
m Converted Pfam box into Protein family infobox |
||
| (16 intermediate revisions by 13 users not shown) | |||
| Line 1: | Line 1: | ||
| ⚫ | |||
{{Pfam_box |
|||
{{Infobox protein family |
|||
| Symbol = Thionin |
| Symbol = Thionin |
||
| Name = Plant thionin |
| Name = Plant thionin |
||
| Line 11: | Line 12: | ||
| SCOP = 1cnb |
| SCOP = 1cnb |
||
| TCDB = 1.C.44 |
| TCDB = 1.C.44 |
||
| OPM family= |
| OPM family= 140 |
||
| OPM protein= 2plh |
| OPM protein= 2plh |
||
| PDB=1BHP |
| PDB=1BHP |
||
}} |
}} |
||
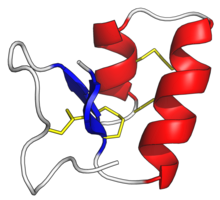
'''Thionins''' are a family of small proteins found solely in [[higher plants]]. Typically, a thionin consists of 45–48 [[amino acid]] residues. 6–8 of these are [[cysteine]] forming 3–4 [[disulfide bonds]]. They include [[phoratoxin]]s and [[viscotoxin]]s. |
|||
| ⚫ | |||
Alpha- and beta- thionins are related to each other. The [[gamma thionin]]s have a superficially similar structure but are an unrelated class of protein, now called plant defensins. |
|||
'''Thionins''' (without an e) are a family of small proteins found solely in [[higher plants]]. Typically, a thionin consists of 45–48 [[amino acid]] residues. 6–8 of these are [[cysteine]] forming 3–4 [[disulfide bonds]]. Some thionins have [[cytotoxic]] activity and they are therefore interesting in the development of new [[drugs]] against [[cancer]] with novel action mechanisms.<ref>{{cite journal |doi=10.1007/BF00039517 |author=Florack DE, Stiekema WJ |title=Thionins: properties, possible biological roles and mechanisms of action |journal=Plant Mol. Biol. |volume=26 |issue=1 |pages=25–37 |date=October 1994 |pmid=7948874 }}</ref> No thionin has yet been developed into an anti-cancer drug. Alpha- and beta- thionins are related to each other. [[Gamma thionin]]s have a similar structure but are an unrelated class of protein, now called plant defensins. |
|||
== Activity == |
|||
The proteins are toxic to animal cells, presumably attacking the cell membrane and rendering it permeable: this results in the inhibition of sugar uptake and allows potassium and phosphate ions, proteins, and nucleotides to leak from cells.<ref name="PUB00000146">{{cite journal | |
The proteins are toxic to animal cells, presumably attacking the cell membrane and rendering it permeable: this results in the inhibition of sugar uptake and allows potassium and phosphate ions, proteins, and nucleotides to leak from cells.<ref name="PUB00000146">{{cite journal |vauthors=Vernon LP, Evett GE, Zeikus RD, Gray WR |title=A toxic thionin from Pyrularia pubera: purification, properties, and amino acid sequence |journal=Arch. Biochem. Biophys. |volume=238 |issue=1 |pages=18–29 |year=1985 |pmid=3985614 |doi=10.1016/0003-9861(85)90136-5}}</ref> Thionins are mainly found in seeds where they may act as a defence against consumption by animals. A barley (''[[Hordeum vulgare]]'') leaf thionin that is highly toxic to plant pathogens and is involved in the mechanism of plant defence against microbial infections has also been identified.<ref name="PUB00004552">{{cite journal |vauthors=Apel K, Andresen I, Becker W, Schluter K, Burges J, Parthier B |title=The identification of leaf thionin as one of the main jasmonate-induced proteins of barley (Hordeum vulgare) |journal=Plant Mol. Biol. |volume=19 |issue=2 |pages=193–204 |year=1992 |pmid=1377959 |doi=10.1007/BF00027341|s2cid=31727379 }}</ref> The hydrophobic protein [[crambin]] from the Abyssinian kale (''[[Crambe abyssinica]]'') is also a member of the thionin family.<ref name="PUB00000146" /> Some thionins have [[cytotoxic]] activity and they are therefore interesting in the development of new [[drugs]] against [[cancer]] with novel action mechanisms.<ref>{{cite journal |doi=10.1007/BF00039517 |vauthors=Florack DE, Stiekema WJ |title=Thionins: properties, possible biological roles and mechanisms of action |journal=Plant Mol. Biol. |volume=26 |issue=1 |pages=25–37 |date=October 1994 |pmid=7948874 |s2cid=5814475 }}</ref> No thionin has yet been developed into an anti-cancer drug. Thionin is also a minor protein found in mustard (Brassica napus L.) seeds.<ref>{{cite journal |author1=Bérot S |author2=Compoint JP |author3=Larré C |author4=Malabat C |author5=Guéguen J.|title=Large scale purification of rapeseed proteins (Brassica napus L.)|doi=10.1016/j.jchromb.2004.08.001 |volume=818 |year=2005 |journal=Journal of Chromatography B |issue=1 |pages=35–42|pmid=15722042 }}</ref> |
||
==Databases== |
==Databases== |
||
A database for antimicrobial peptides, including thionins is available: PhytAMP.<ref name="pmid18836196">{{cite web | url = http://phytamp. |
A database for antimicrobial peptides, including thionins is available: PhytAMP.<ref name="pmid18836196">{{cite web | url = http://phytamp.hammamilab.org | title = PhytAMP Database }}; {{cite journal | vauthors = Hammami R, Ben Hamida J, Vergoten G, Fliss I | title = PhytAMP: a database dedicated to antimicrobial plant peptides | journal = Nucleic Acids Res. | volume = 37 | issue = Database issue | pages = D963–8 |date=January 2009 | pmid = 18836196 | pmc = 2686510 | doi = 10.1093/nar/gkn655 }}</ref> |
||
== See also == |
== See also == |
||
| Line 37: | Line 39: | ||
[[Category:Peripheral membrane proteins]] |
[[Category:Peripheral membrane proteins]] |
||
[[Category:Plant toxins]] |
[[Category:Plant toxins]] |
||
[[Category:Antimicrobial peptides]] |
|||
{{biochem-stub}} |
|||
Latest revision as of 15:11, 1 October 2024
| Plant thionin | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||
| Identifiers | |||||||||||
| Symbol | Thionin | ||||||||||
| Pfam | PF00321 | ||||||||||
| InterPro | IPR001010 | ||||||||||
| PROSITE | PDOC00244 | ||||||||||
| SCOP2 | 1cnb / SCOPe / SUPFAM | ||||||||||
| TCDB | 1.C.44 | ||||||||||
| OPM superfamily | 140 | ||||||||||
| OPM protein | 2plh | ||||||||||
| |||||||||||
Thionins are a family of small proteins found solely in higher plants. Typically, a thionin consists of 45–48 amino acid residues. 6–8 of these are cysteine forming 3–4 disulfide bonds. They include phoratoxins and viscotoxins.
Alpha- and beta- thionins are related to each other. The gamma thionins have a superficially similar structure but are an unrelated class of protein, now called plant defensins.
Activity
[edit]The proteins are toxic to animal cells, presumably attacking the cell membrane and rendering it permeable: this results in the inhibition of sugar uptake and allows potassium and phosphate ions, proteins, and nucleotides to leak from cells.[1] Thionins are mainly found in seeds where they may act as a defence against consumption by animals. A barley (Hordeum vulgare) leaf thionin that is highly toxic to plant pathogens and is involved in the mechanism of plant defence against microbial infections has also been identified.[2] The hydrophobic protein crambin from the Abyssinian kale (Crambe abyssinica) is also a member of the thionin family.[1] Some thionins have cytotoxic activity and they are therefore interesting in the development of new drugs against cancer with novel action mechanisms.[3] No thionin has yet been developed into an anti-cancer drug. Thionin is also a minor protein found in mustard (Brassica napus L.) seeds.[4]
Databases
[edit]A database for antimicrobial peptides, including thionins is available: PhytAMP.[5]
See also
[edit]References
[edit]- ^ a b Vernon LP, Evett GE, Zeikus RD, Gray WR (1985). "A toxic thionin from Pyrularia pubera: purification, properties, and amino acid sequence". Arch. Biochem. Biophys. 238 (1): 18–29. doi:10.1016/0003-9861(85)90136-5. PMID 3985614.
- ^ Apel K, Andresen I, Becker W, Schluter K, Burges J, Parthier B (1992). "The identification of leaf thionin as one of the main jasmonate-induced proteins of barley (Hordeum vulgare)". Plant Mol. Biol. 19 (2): 193–204. doi:10.1007/BF00027341. PMID 1377959. S2CID 31727379.
- ^ Florack DE, Stiekema WJ (October 1994). "Thionins: properties, possible biological roles and mechanisms of action". Plant Mol. Biol. 26 (1): 25–37. doi:10.1007/BF00039517. PMID 7948874. S2CID 5814475.
- ^ Bérot S; Compoint JP; Larré C; Malabat C; Guéguen J. (2005). "Large scale purification of rapeseed proteins (Brassica napus L.)". Journal of Chromatography B. 818 (1): 35–42. doi:10.1016/j.jchromb.2004.08.001. PMID 15722042.
- ^ "PhytAMP Database".; Hammami R, Ben Hamida J, Vergoten G, Fliss I (January 2009). "PhytAMP: a database dedicated to antimicrobial plant peptides". Nucleic Acids Res. 37 (Database issue): D963–8. doi:10.1093/nar/gkn655. PMC 2686510. PMID 18836196.
