4-PrO-DMT: Difference between revisions
No edit summary |
|||
| (23 intermediate revisions by 15 users not shown) | |||
| Line 18: | Line 18: | ||
}} |
}} |
||
'''4-Propionoxy-N,N-dimethyltryptamine''' ('''4-PrO-DMT''', or '''O-Propionylpsilocin''') is a synthetic [[psychedelic drug]] from the [[tryptamine]] family with [[psychedelic]] effects, and is believed to act as a [[prodrug]] for [[psilocin]]. |
'''4-Propionoxy-''N,N''-dimethyltryptamine''' ('''4-PrO-DMT''', or '''O-Propionylpsilocin''') is a synthetic [[psychedelic drug]] from the [[tryptamine]] family with [[psychedelic]] effects, and is believed to act as a [[prodrug]] for [[psilocin]].<ref name = "Glatfelter_2023">{{cite journal | vauthors = Glatfelter GC, Naeem M, Pham DN, Golen JA, Chadeayne AR, Manke DR, Baumann MH | title = Receptor Binding Profiles for Tryptamine Psychedelics and Effects of 4-Propionoxy-''N,N''-dimethyltryptamine in Mice | journal = ACS Pharmacology & Translational Science | volume = 6 | issue = 4 | pages = 567–577 | date = April 2023 | pmid = 37082754 | pmc = 10111620 | doi = 10.1021/acsptsci.2c00222 }}</ref> It produces a [[head-twitch response]] in mice.<ref name = "Glatfelter_2023" /> It has been sold online as a [[designer drug]] since May 2019. It was first identified as a new [[Psychoactive drug|psychoactive substance]] in Sweden, in July 2019.<ref>{{cite book | url = https://www.emcdda.europa.eu/system/files/publications/13464/20205648_TD0320796ENN_PDF_rev.pdf | title = New psychoactive substances: global markets, glocal threats and the COVID-19 pandemic. An update from the EU Early Warning System | author = European Monitoring Center for Drugs and Drug Addiction | location = Luxembourg | publisher = Publications Office of the European Union | date = December 2020 | doi = 10.2810/921262 | isbn = 9789294975584 | access-date = 2021-06-17 | archive-date = 2022-10-08 | archive-url = https://web.archive.org/web/20221008161408/http://www.emcdda.europa.eu/system/files/publications/13464/20205648_TD0320796ENN_PDF_rev.pdf | url-status = live }}</ref> A number of related derivatives have been synthesized as [[prodrug]]s of [[psilocin]] for medical applications.<ref name="pmid37983270">{{cite journal | vauthors = Raithatha SA, Hagel JM, Matinkhoo K, Yu L, Press D, Cook SG, Sharma G, Dhananjaya D, Jensen G, Lee JB, Cai C, Gallant J, Bains J, Tucker JE, Facchini PJ | display-authors = 6 | title = Novel Psilocin Prodrugs with Altered Pharmacological Properties as Candidate Therapies for Treatment-Resistant Anxiety Disorders | journal = Journal of Medicinal Chemistry | volume = 67| issue = 2| date = November 2023 | pages = 1024–1043 | pmid = 37983270 | doi = 10.1021/acs.jmedchem.3c01225 | doi-access = free | pmc = 10823477 }}</ref> |
||
== Recreational use == |
== Recreational use == |
||
| Line 24: | Line 24: | ||
=== Dosage === |
=== Dosage === |
||
4-PrO-DMT is reported to be orally |
4-PrO-DMT is reported to be orally [[Psychoactive drug|psychoactive substance]] and while dosage effects have been studied in mice, its effects and longevity on humans has not been formally studied. The effects of 4-PrO-DMT are similar to those of [[psilocin]] (4-HO-DMT), as it acts as a [[prodrug]].<ref name="Glatfelter_2023" /><ref>{{Cite web |title=Foundational Science for the Psilocybin Analog 4-PrO-DMT in Mice |url=https://caam.tech/foundational-science-for-the-psilocybin-analog-4-pro-dmt-in-mice/ |url-status=live |website=CaaMTech |date=28 March 2023 |archive-url=https://web.archive.org/web/20240303000330/https://caam.tech/foundational-science-for-the-psilocybin-analog-4-pro-dmt-in-mice/|archive-date=2024-03-03 }}</ref> |
||
=== Effects === |
|||
The effects of 4-PrO-DMT are broadly comparable to those of other serotonergic psychedelics such as [[LSD]] and [[psilocybin]]. |
|||
<ref>{{cite journal | vauthors = Andersen KA, Carhart-Harris R, Nutt DJ, Erritzoe D | title = Therapeutic effects of classic serotonergic psychedelics: A systematic review of modern-era clinical studies | journal = Acta Psychiatrica Scandinavica | volume = 143 | issue = 2 | pages = 101–118 | date = February 2021 | pmid = 33125716 | doi = 10.1111/acps.13249 | s2cid = 226217912 }}</ref> |
|||
== Pharmacology == |
== Pharmacology == |
||
===Pharmacodynamics=== |
===Pharmacodynamics=== |
||
4-PrO-DMT is theorized to be a [[serotonergic]] [[Psychedelic drug|psychedelic]], and is [[partial agonist]] of the [[5-HT1D receptor|5-HT<sub>1D</sub>]], [[5-HT1B receptor|5-HT<sub>1B</sub>]] and [[5-HT1A receptor|5-HT<sub>1A</sub>]] [[5-HT receptor|serotonin receptors]].<ref>{{ |
4-PrO-DMT is theorized to be a [[Serotonin|serotonergic]] [[Psychedelic drug|psychedelic]], and is [[partial agonist]] of the [[5-HT1D receptor|5-HT<sub>1D</sub>]], [[5-HT1B receptor|5-HT<sub>1B</sub>]] and [[5-HT1A receptor|5-HT<sub>1A</sub>]] [[5-HT receptor|serotonin receptors]].<ref name="Glatfelter_2023" /><ref>{{Citation |last=Greene |first=Shaun L. |title=Chapter18 - Tryptamines |date=2022-01-01 |work=Novel Psychoactive Substances (Second Edition) |pages=495–532 |editor-last=Dargan |editor-first=Paul |url=https://linkinghub.elsevier.com/retrieve/pii/B9780128187883000140 |access-date=2024-08-29 |place=Boston |publisher=Academic Press |doi=10.1016/b978-0-12-818788-3.00014-0 |isbn=978-0-12-818788-3 |editor2-last=Wood |editor2-first=David}}</ref> |
||
Similar to [[4-AcO-DMT]], 4-PrO-DMT is believed to be a [[pro-drug]] of [[psilocin]], as well as showing some direct serotonin receptor agonist effects in its own right.<ref>{{cite journal | vauthors = Mashkovsky MD, Yakhontov LN | title = Relationships between the chemical structure and pharmacological activity in a series of synthetic quinuclidine derivatives | journal = Progress in Drug Research. Fortschritte der Arzneimittelforschung. Progres des Recherches Pharmaceutiques | volume = 13 | pages = 293–339 | date = 1969 | pmid = 4391190 | doi = 10.1007/978-3-0348-7068-9_6 | isbn = 978-3-0348-7070-2 }}</ref> |
|||
=== Toxicity === |
=== Toxicity === |
||
Very little data about the toxicity or pharmacology of 4-PrO-DMT is known. Its chemical structure and pharmacological activity are similar to [[psilacetin]], a compound which isn't associated with compulsive use or physical dependence. However, due to lack of research and data, it cannot be definitively concluded that its pharmacological actions in the human body do not differ from those of psilacetin. To date, there have been no reported deaths from 4-PrO-DMT. |
Very little data about the [[toxicity]] or [[pharmacology]] of 4-PrO-DMT is known. Its chemical structure and [[Pharmacology|pharmacological]] activity are similar to [[psilacetin]], a compound which isn't associated with compulsive use or physical dependence. However, due to lack of research and data, it cannot be definitively concluded that its pharmacological actions in the human body do not differ from those of [[psilacetin]]. To date, there have been no reported deaths from 4-PrO-DMT.<ref name="Glatfelter_2023" /> |
||
== See also == |
== See also == |
||
* [[4-HO-DMT]] |
* [[4-HO-DMT]] |
||
* [[4-AcO-DMT]] |
* [[4-AcO-DMT]] |
||
*[[5-MeO-DMT]] |
* [[5-MeO-DMT]] |
||
* [[FT-104]] |
|||
* [[Psilocybin]] |
* [[Psilocybin]] |
||
| Line 61: | Line 55: | ||
[[Category:Designer drugs]] |
[[Category:Designer drugs]] |
||
[[Category:Esters]] |
[[Category:Esters]] |
||
{{nervous-system-drug-stub}} |
|||
Latest revision as of 15:30, 1 October 2024
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 4-Propanoyloxy dmt |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
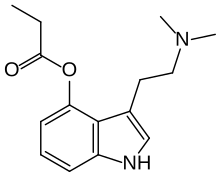
| Formula | C15H20N2O2 |
| Molar mass | 260.337 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
4-Propionoxy-N,N-dimethyltryptamine (4-PrO-DMT, or O-Propionylpsilocin) is a synthetic psychedelic drug from the tryptamine family with psychedelic effects, and is believed to act as a prodrug for psilocin.[1] It produces a head-twitch response in mice.[1] It has been sold online as a designer drug since May 2019. It was first identified as a new psychoactive substance in Sweden, in July 2019.[2] A number of related derivatives have been synthesized as prodrugs of psilocin for medical applications.[3]
Recreational use
[edit]
Dosage
[edit]4-PrO-DMT is reported to be orally psychoactive substance and while dosage effects have been studied in mice, its effects and longevity on humans has not been formally studied. The effects of 4-PrO-DMT are similar to those of psilocin (4-HO-DMT), as it acts as a prodrug.[1][4]
Pharmacology
[edit]Pharmacodynamics
[edit]4-PrO-DMT is theorized to be a serotonergic psychedelic, and is partial agonist of the 5-HT1D, 5-HT1B and 5-HT1A serotonin receptors.[1][5]
Toxicity
[edit]Very little data about the toxicity or pharmacology of 4-PrO-DMT is known. Its chemical structure and pharmacological activity are similar to psilacetin, a compound which isn't associated with compulsive use or physical dependence. However, due to lack of research and data, it cannot be definitively concluded that its pharmacological actions in the human body do not differ from those of psilacetin. To date, there have been no reported deaths from 4-PrO-DMT.[1]
See also
[edit]References
[edit]- ^ a b c d e Glatfelter GC, Naeem M, Pham DN, Golen JA, Chadeayne AR, Manke DR, Baumann MH (April 2023). "Receptor Binding Profiles for Tryptamine Psychedelics and Effects of 4-Propionoxy-N,N-dimethyltryptamine in Mice". ACS Pharmacology & Translational Science. 6 (4): 567–577. doi:10.1021/acsptsci.2c00222. PMC 10111620. PMID 37082754.
- ^ European Monitoring Center for Drugs and Drug Addiction (December 2020). New psychoactive substances: global markets, glocal threats and the COVID-19 pandemic. An update from the EU Early Warning System (PDF). Luxembourg: Publications Office of the European Union. doi:10.2810/921262. ISBN 9789294975584. Archived (PDF) from the original on 2022-10-08. Retrieved 2021-06-17.
- ^ Raithatha SA, Hagel JM, Matinkhoo K, Yu L, Press D, Cook SG, et al. (November 2023). "Novel Psilocin Prodrugs with Altered Pharmacological Properties as Candidate Therapies for Treatment-Resistant Anxiety Disorders". Journal of Medicinal Chemistry. 67 (2): 1024–1043. doi:10.1021/acs.jmedchem.3c01225. PMC 10823477. PMID 37983270.
- ^ "Foundational Science for the Psilocybin Analog 4-PrO-DMT in Mice". CaaMTech. 28 March 2023. Archived from the original on 2024-03-03.
- ^ Greene, Shaun L. (2022-01-01), Dargan, Paul; Wood, David (eds.), "Chapter18 - Tryptamines", Novel Psychoactive Substances (Second Edition), Boston: Academic Press, pp. 495–532, doi:10.1016/b978-0-12-818788-3.00014-0, ISBN 978-0-12-818788-3, retrieved 2024-08-29
