2,5-Dimethoxy-4-propylamphetamine: Difference between revisions
Appearance
Content deleted Content added
Chemical formula was wrong. |
No edit summary |
||
| (25 intermediate revisions by 20 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description|Psychedelic drug}} |
|||
{{one source|date=April 2015}} |
|||
{{Drugbox |
{{Drugbox |
||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Watchedfields = changed |
| Watchedfields = changed |
||
| verifiedrevid = 477211774 |
| verifiedrevid = 477211774 |
||
| IUPAC_name = 1-(2,5- |
| IUPAC_name = 1-(2,5-Dimethoxy-4-propylphenyl)propan-2-amine |
||
| image = 2,5-Dimethoxy-4-propylamphetamine.svg |
| image = 2,5-Dimethoxy-4-propylamphetamine.svg |
||
| width = |
| width = 200px |
||
<!--Clinical data--> |
<!--Clinical data--> |
||
| tradename = |
| tradename = |
||
| pregnancy_category = |
| pregnancy_category = |
||
| legal_CA = Schedule I |
|||
| legal_DE = NpSG |
|||
| legal_UK = Class A |
|||
| legal_status = |
| legal_status = |
||
| routes_of_administration = |
| routes_of_administration = |
||
<!--Pharmacokinetic data--> |
<!--Pharmacokinetic data--> |
||
| Line 18: | Line 23: | ||
| metabolism = |
| metabolism = |
||
| elimination_half-life = |
| elimination_half-life = |
||
| excretion = |
| excretion = |
||
<!--Identifiers--> |
<!--Identifiers--> |
||
| Line 24: | Line 29: | ||
| CAS_number = 63779-88-4 |
| CAS_number = 63779-88-4 |
||
| CAS_supplemental = <BR> 53581-55-8 (hydrochloride) |
| CAS_supplemental = <BR> 53581-55-8 (hydrochloride) |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = L3P287BI9W |
|||
| ATC_prefix = |
| ATC_prefix = |
||
| ATC_suffix = |
| ATC_suffix = |
||
| Line 36: | Line 43: | ||
<!--Chemical data--> |
<!--Chemical data--> |
||
| C=14 | H=23 | N=1 | O=2 |
| C=14 | H=23 | N=1 | O=2 |
||
| molecular_weight = 237.34 g/mol |
|||
| smiles = O(c1cc(c(OC)cc1CC(N)C)CCC)C |
| smiles = O(c1cc(c(OC)cc1CC(N)C)CCC)C |
||
| ⚫ | |||
| InChI = 1/C14H23NO2/c1-5-6-11-8-14(17-4)12(7-10(2)15)9-13(11)16-3/h8-10H,5-7,15H2,1-4H3 |
|||
| InChIKey = UEEAUFJYLUJWQJ-UHFFFAOYAM |
|||
| ⚫ | |||
| StdInChI = 1S/C15H25NO2/c1-5-6-11-8-14(17-4)12(7-10(2)15)9-13(11)16-3/h8-10H,5-7,15H2,1-4H3 |
| StdInChI = 1S/C15H25NO2/c1-5-6-11-8-14(17-4)12(7-10(2)15)9-13(11)16-3/h8-10H,5-7,15H2,1-4H3 |
||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
||
| StdInChIKey = UEEAUFJYLUJWQJ-UHFFFAOYSA-N |
| StdInChIKey = UEEAUFJYLUJWQJ-UHFFFAOYSA-N |
||
| synonyms = 2,5- |
| synonyms = 2,5-Dimethoxy-4-propylamphetamine |
||
}} |
}} |
||
'''2,5-Dimethoxy-4-propylamphetamine''' ('''DOPR''') is a [[ |
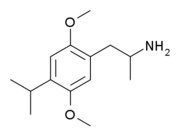
'''2,5-Dimethoxy-4-propylamphetamine''' ('''DOPR''') is a [[psychedelic drug|psychedelic]] [[drug]] of the [[substituted phenethylamine|phenethylamine]] and [[substituted amphetamine|amphetamine]] [[chemical class]]es. It was first [[Organic synthesis|synthesized]] by [[Alexander Shulgin]], and was described in his book ''[[PiHKAL]]'' (''Phenethylamines i Have Known And Loved''). Shulgin described DOPR as a "heavy duty psychedelic", complete with alterations of the thought process and visual distortion.<ref name="PiHKAL">{{cite book | title = PiHKAL: A Chemical Love Story | vauthors = Shulgin A, Shulgin A | publisher = Transform Press | location = United States | isbn = 0-9630096-0-5 | pages = 978 | url = http://www.erowid.org/library/books_online/pihkal/pihkal.shtml |date= September 1991}}</ref> Very little data exists about the pharmacological properties, metabolism, and toxicity of DOPR. |
||
The alternative structural isomer DOIP, with a 4-isopropyl substitution, is also known but is around ten times weaker than DOPR, with an active dose of some |
The alternative structural isomer [[2,5-Dimethoxy-4-isopropylamphetamine|DOIP]], with a 4-isopropyl substitution, is also known but is around ten times weaker than DOPR, with an active dose of some 20–30 mg (as compared to 2–5 mg for DOPR).<ref name="PiHKAL"/> |
||
[[Image:DOIP_structure.png|180px|thumb|left|Structure of DOIP]]{{clear-left}} |
[[Image:DOIP_structure.png|180px|thumb|left|Structure of DOIP]]{{clear-left}} |
||
== See also == |
== See also == |
||
* [[DOPF]] |
|||
* [[2,5-Dimethoxy-4-Substituted Amphetamine]]s |
|||
* [[DOx]] |
|||
== References == |
== References == |
||
{{Reflist |
{{Reflist}} |
||
== External links == |
|||
* [http://www.erowid.org/library/books_online/pihkal/pihkal071.shtml DOPR Entry in ''PiHKAL''] |
|||
* [http://pihkal.info/read.php?domain=pk&id=71 DOPR Entry in PiHKAL • info] |
|||
{{Hallucinogens}} |
{{Hallucinogens}} |
||
{{Serotonergics}} |
{{Serotonergics}} |
||
{{Phenethylamines}} |
{{Phenethylamines}} |
||
| ⚫ | |||
[[Category:2C (psychedelics)]] |
|||
{{DEFAULTSORT:Dimethoxy-4-propylamphetamine, 2,5-}} |
{{DEFAULTSORT:Dimethoxy-4-propylamphetamine, 2,5-}} |
||
[[Category:Substituted amphetamines]] |
|||
| ⚫ | |||
{{ |
{{hallucinogen-stub}} |
||
Latest revision as of 15:42, 5 October 2024
This article relies largely or entirely on a single source. (April 2015) |
 | |
| Clinical data | |
|---|---|
| Other names | 2,5-Dimethoxy-4-propylamphetamine |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H23NO2 |
| Molar mass | 237.343 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
2,5-Dimethoxy-4-propylamphetamine (DOPR) is a psychedelic drug of the phenethylamine and amphetamine chemical classes. It was first synthesized by Alexander Shulgin, and was described in his book PiHKAL (Phenethylamines i Have Known And Loved). Shulgin described DOPR as a "heavy duty psychedelic", complete with alterations of the thought process and visual distortion.[1] Very little data exists about the pharmacological properties, metabolism, and toxicity of DOPR.
The alternative structural isomer DOIP, with a 4-isopropyl substitution, is also known but is around ten times weaker than DOPR, with an active dose of some 20–30 mg (as compared to 2–5 mg for DOPR).[1]
See also
[edit]References
[edit]- ^ a b Shulgin A, Shulgin A (September 1991). PiHKAL: A Chemical Love Story. United States: Transform Press. p. 978. ISBN 0-9630096-0-5.
