Propyl benzoate: Difference between revisions
Appearance
Content deleted Content added
m moved User:Shoy/Propyl benzoate to Propyl benzoate: Moving to mainspace |
|||
| (45 intermediate revisions by 32 users not shown) | |||
| Line 1: | Line 1: | ||
{{chembox |
{{chembox |
||
| Verifiedfields = changed |
|||
| Watchedfields = changed |
|||
| verifiedrevid = 464217147 |
|||
| Name = Propyl benzoate |
| Name = Propyl benzoate |
||
| ImageFile = |
| ImageFile = Propyl benzoate.svg |
||
| ImageSize = |
| ImageSize = |
||
| ImageName = |
| ImageName = Propyl benzoate |
||
| |
| PIN = Propyl benzoate |
||
| OtherNames = ''n''-propyl benzoate, benzoic acid propyl ester |
| OtherNames = ''n''-propyl benzoate, benzoic acid propyl ester |
||
| |
|Section1={{Chembox Identifiers |
||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| ChemSpiderID = 15965 |
|||
| PubChem = 16846 |
|||
| ChEBI = 156072 |
|||
| InChI = 1/C10H12O2/c1-2-8-12-10(11)9-6-4-3-5-7-9/h3-7H,2,8H2,1H3 |
|||
| InChIKey = UDEWPOVQBGFNGE-UHFFFAOYAH |
|||
| SMILES = O=C(OCCC)c1ccccc1 |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChI = 1S/C10H12O2/c1-2-8-12-10(11)9-6-4-3-5-7-9/h3-7H,2,8H2,1H3 |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = UDEWPOVQBGFNGE-UHFFFAOYSA-N |
|||
| CASNo = 2315-68-6 |
| CASNo = 2315-68-6 |
||
| CASNo_Ref = |
| CASNo_Ref = {{cascite|correct|CAS}} |
||
| |
| CASNoOther = |
||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| ⚫ | |||
| UNII = VWK210B7WS |
|||
| ⚫ | |||
| EINECS = 219-020-8 |
| EINECS = 219-020-8 |
||
}} |
}} |
||
| |
|Section2={{Chembox Properties |
||
| Formula = C<sub>10</sub>H<sub>12</sub>O<sub>2</sub> |
| Formula = C<sub>10</sub>H<sub>12</sub>O<sub>2</sub> |
||
| MolarMass = 164.201 g/mol |
| MolarMass = 164.201 g/mol |
||
| Appearance = colorless oily liquid, nutty odor |
| Appearance = colorless oily liquid, nutty odor |
||
| Density = 1.0230 g/cm<sup>3</sup> at 20°C |
| Density = 1.0230 g/cm<sup>3</sup> at 20 °C |
||
| MagSus = -105.00·10<sup>−6</sup> cm<sup>3</sup>/mol |
|||
| MeltingPt = -51.6°C |
|||
| MeltingPtC = −51.6 |
|||
| ⚫ | |||
| BoilingPtC = 230 |
|||
| ⚫ | |||
| Solubility = insoluble |
| Solubility = insoluble |
||
| SolubleOther = miscible with [[ethanol]], [[diethyl ether]]<ref name="hand"> |
| SolubleOther = miscible with [[ethanol]], [[diethyl ether]]<ref name="hand"> |
||
{{Cite book |
|||
{{Citation |
|||
| last = Lide |
|||
| first = David R. |
|||
| author-link = |
|||
| ⚫ | |||
| last2 = |
|||
| year = 1998 |
|||
| ⚫ | |||
| author2-link = |
|||
| edition = 87 |
|||
| ⚫ | |||
| volume = |
|||
| series = |
|||
| ⚫ | |||
| ⚫ | |||
| place = |
|||
| publisher = CRC Press |
|||
| id = |
|||
| isbn = 0-8493-0594-2 |
|||
| ⚫ | |||
| doi = |
|||
| ⚫ | |||
| ⚫ | |||
| pages = 3–484 |
|||
| ⚫ | |||
| isbn = 0849305942 |
|||
| accessdate = |
|||
| ⚫ | |||
| pages = 3-484 |
|||
| ⚫ | |||
| accessdate = |
|||
}}</ref> |
}}</ref> |
||
}} |
}} |
||
| |
|Section3={{Chembox Structure |
||
| Coordination = |
| Coordination = |
||
| CrystalStruct = |
| CrystalStruct = |
||
}} |
}} |
||
| |
|Section4={{Chembox Thermochemistry |
||
| DeltaHf = |
| DeltaHf = |
||
| DeltaHc = |
| DeltaHc = |
||
| Entropy = |
| Entropy = |
||
| HeatCapacity = |
| HeatCapacity = |
||
}} |
}} |
||
| |
|Section7={{Chembox Hazards |
||
| ExternalSDS = [https://fscimage.fishersci.com/msds/00105.htm External MSDS] |
|||
| ExternalMSDS = |
|||
| |
| NFPA-H = |
||
| NFPA-H = |
|||
| NFPA-F = |
| NFPA-F = |
||
| NFPA-R = |
| NFPA-R = |
||
| |
| HPhrases = |
||
| |
| PPhrases = |
||
| GHS_ref = |
|||
| ⚫ | |||
| FlashPtC = 98 |
|||
| ⚫ | |||
}} |
}} |
||
| |
|Section8={{Chembox Related |
||
| OtherCompounds = [[Methyl benzoate]]<br/>[[Ethyl benzoate]] |
|||
| OtherAnions = |
|||
| OtherCations = |
|||
}} |
}} |
||
}} |
}} |
||
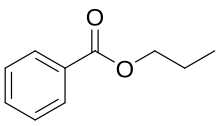
'''Propyl benzoate''' is an [[organic chemistry|organic]] [[chemical compound]] used as a [[food additive]]. |
'''Propyl benzoate''' is an [[organic chemistry|organic]] [[chemical compound]] used as a [[food additive]]. It is an [[ester]]. |
||
==Uses== |
==Uses== |
||
Propyl benzoate has a nutty odor and sweet fruity or nut-like taste, and as such, it is used as a synthetic flavoring agent in foods. It also has [[antimicrobial]] properties and is used as a preservative in [[cosmetics]]. It occurs naturally in the [[sweet cherry]] |
Propyl benzoate has a nutty odor and sweet fruity or nut-like taste, and as such, it is used as a synthetic flavoring agent in foods. It also has [[antimicrobial]] properties and is used as a preservative in [[cosmetics]]. It occurs naturally in the [[sweet cherry]] and in [[clove]] [[Plant stem|stems]], as well as in [[butter]].<ref name="BookPreserv">{{cite book |last=Ash |first=Michael |author2=Ash, Irene |title=Handbook of Preservatives |url=https://books.google.com/books?id=XZ2QB7bu5LwC&pg=PA404 |accessdate=2009-05-04 |year=2004 |publisher=Synapse Information Resources |isbn=1-890595-66-7 |page=508}}</ref><ref name="encyc">{{cite book |
||
| last = Burdock |
|||
| first = George A. |
|||
| authorlink = |
|||
| ⚫ | |||
| coauthors = |
|||
| ⚫ | |||
| ⚫ | |||
| date = 1997 |
|||
| publisher = CRC Press |
|||
| location = |
|||
| pages = 2340 |
|||
| ⚫ | |||
| pages = 2340 |
|||
| doi = |
|||
| ⚫ | |||
| id = |
|||
| ⚫ | |||
| id = |
|||
| ⚫ | |||
==Reactions== |
==Reactions== |
||
Propyl benzoate can be synthesized by the [[ |
Propyl benzoate can be synthesized by the [[transesterification]] of [[methyl benzoate]] with [[propanol]].<ref name="encyc"/> |
||
Propyl benzoate can also be synthesized by means of [[Fischer esterification]] of [[benzoic acid]] with [[propanol]]. |
|||
==References== |
==References== |
||
{{Reflist}} |
{{Reflist}} |
||
<!-- {{organic-compound-stub}} |
|||
[[Category:Food additives]] |
[[Category:Food additives]] |
||
[[Category: |
[[Category:Preservatives]] |
||
[[Category: |
[[Category:Benzoate esters]] |
||
[[Category:Preservatives]] --> |
|||
{{ester-stub}}{{Esters}} |
|||
Latest revision as of 16:02, 12 October 2024
| |
| Names | |
|---|---|
| Preferred IUPAC name
Propyl benzoate | |
| Other names
n-propyl benzoate, benzoic acid propyl ester
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.017.292 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H12O2 | |
| Molar mass | 164.201 g/mol |
| Appearance | colorless oily liquid, nutty odor |
| Density | 1.0230 g/cm3 at 20 °C |
| Melting point | −51.6 °C (−60.9 °F; 221.6 K) |
| Boiling point | 230 °C (446 °F; 503 K)[2] |
| insoluble | |
| Solubility | miscible with ethanol, diethyl ether[1] |
| -105.00·10−6 cm3/mol | |
| Hazards | |
| Flash point | 98 °C (208 °F; 371 K)[2] |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related compounds
|
Methyl benzoate Ethyl benzoate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Propyl benzoate is an organic chemical compound used as a food additive. It is an ester.
Uses
[edit]Propyl benzoate has a nutty odor and sweet fruity or nut-like taste, and as such, it is used as a synthetic flavoring agent in foods. It also has antimicrobial properties and is used as a preservative in cosmetics. It occurs naturally in the sweet cherry and in clove stems, as well as in butter.[2][3]
Reactions
[edit]Propyl benzoate can be synthesized by the transesterification of methyl benzoate with propanol.[3] Propyl benzoate can also be synthesized by means of Fischer esterification of benzoic acid with propanol.
References
[edit]- ^ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, Florida: CRC Press. pp. 3–484. ISBN 0-8493-0594-2.
- ^ a b c Ash, Michael; Ash, Irene (2004). Handbook of Preservatives. Synapse Information Resources. p. 508. ISBN 1-890595-66-7. Retrieved 2009-05-04.
- ^ a b Burdock, George A. (1997). Encyclopedia of Food and Color Additives. CRC Press. p. 2340. ISBN 978-0-8493-9416-4.
