Onternabez: Difference between revisions
No edit summary |
Slothwizard (talk | contribs) No edit summary Tags: Visual edit Mobile edit Mobile web edit |
||
| (44 intermediate revisions by 19 users not shown) | |||
| Line 1: | Line 1: | ||
{{short description| |
{{short description|Cannabidiol-derivative drug}} |
||
{{Copy edit|for=Capitalization, name-dropping of individuals by last name who are not universally known by readers|date=February 2021}} |
|||
{{Drugbox |
{{Drugbox |
||
| Verifiedfields |
| Verifiedfields = changed |
||
| Watchedfields |
| Watchedfields = changed |
||
| class = [[CB2 receptor|CB<sub>2</sub> receptor agonist]] |
|||
| verifiedrevid = 426291380 |
|||
| verifiedrevid = 426291380 |
|||
| IUPAC_name = [(1''R'',2''R'',5''R'')-2-[2,6-Dimethoxy-4-(2-methyloctan-2-yl)phenyl]-7,7-dimethyl-4-bicyclo[3.1.1]hept-3-enyl]methanol |
|||
| IUPAC_name = [(1''S'',2''S'',5''S'')-2-[2,6-Dimethoxy-4-(2-methyloctan-2-yl)phenyl]-7,7-dimethyl-4-bicyclo[3.1.1]hept-3-enyl]methanol |
|||
| image = HU-308.png |
|||
| image = Onternabez.svg |
|||
| caption = |
|||
| caption = |
|||
| width = |
|||
| width = <!--Clinical data--> |
|||
| tradename = |
|||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|||
| pregnancy_category = |
|||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> |
|||
| legal_CA = Schedule II |
|||
| legal_UK = Class B |
|||
| legal_US = Unscheduled <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> <BR> |
|||
| legal_US_comment = |
|||
| legal_status = Florida: Schedule I |
|||
| routes_of_administration = <!--Pharmacokinetic data--> |
|||
| bioavailability = |
|||
| protein_bound = |
|||
| metabolism = [[Liver]] |
|||
| elimination_half-life = |
|||
| excretion = Kidneys |
|||
<!--Identifiers-->| CAS_number_Ref = {{cascite|correct|CAS}} |
|||
<!--Clinical data--> |
|||
| CAS_number = 256934-39-1 |
|||
| tradename = |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|||
| UNII = 8I5L034D55 |
|||
| pregnancy_category = |
|||
| ATC_prefix = None |
|||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> |
|||
| ATC_suffix = |
|||
| legal_CA = Schedule II |
|||
| PubChem = 11553430 |
|||
| legal_UK = Class B |
|||
| ChEBI = 146244 |
|||
| legal_US = Unscheduled <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> <BR> |
|||
| synonyms = HU-308, HU308, PPP-003, ARDS-003 |
|||
| legal_US_comment = |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| legal_status = Florida: Schedule I |
|||
| DrugBank = |
|||
| routes_of_administration = injection, oral, eyedrops |
|||
| KEGG = D12305 |
|||
| ChemSpiderID = 8020425 |
|||
<!--Pharmacokinetic data--> |
|||
<!--Chemical data-->| C = 27 |
|||
| bioavailability = |
|||
| H = 42 |
|||
| protein_bound = |
|||
| O = 3 |
|||
| metabolism = [[Liver]] |
|||
| smiles = CCCCCCC(C)(C)C1=CC(=C(C(=C1)OC)[C@H]2C=C([C@@H]3C[C@H]2C3(C)C)CO)OC |
|||
| elimination_half-life = |
|||
| StdInChI = 1S/C27H42O3/c1-8-9-10-11-12-26(2,3)19-14-23(29-6)25(24(15-19)30-7)20-13-18(17-28)21-16-22(20)27(21,4)5/h13-15,20-22,28H,8-12,16-17H2,1-7H3/t20-,21-,22+/m0/s1 |
|||
| excretion = Kidneys |
|||
| StdInChIKey = CFMRIVODIXTERW-FDFHNCONSA-N |
|||
<!--Identifiers--> |
|||
| CAS_number_Ref = {{cascite|changed|CAS}} |
|||
| CAS_number = 256934-39-1 |
|||
| UNII_Ref = {{fdacite|changed|FDA}} |
|||
| UNII = 8I5L034D55 |
|||
| ATC_prefix = |
|||
| ATC_suffix = |
|||
| PubChem = 5311172 |
|||
| ChEBI = 146244 |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| DrugBank = |
|||
| ChemSpiderID = 8020425 |
|||
<!--Chemical data--> |
|||
| C = 27 | H = 42 | O = 3 |
|||
| smiles = CCCCCCC(C)(C)C1=CC(=C(C(=C1)OC)[C@H]2C=C([C@@H]3C[C@H]2C3(C)C)CO)OC |
|||
| StdInChI = 1S/C27H42O3/c1-8-9-10-11-12-26(2,3)19-14-23(29-6)25(24(15-19)30-7)20-13-18(17-28)21-16-22(20)27(21,4)5/h13-15,20-22,28H,8-12,16-17H2,1-7H3/t20-,21-,22+/m0/s1 |
|||
| StdInChIKey = CFMRIVODIXTERW-FDFHNCONSA-N |
|||
}} |
}} |
||
'''HU-308''', |
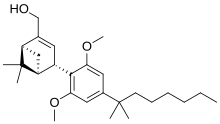
'''Onternabez''' (also known as '''HU-308''', '''HU308''', '''PPP-003''', and '''ARDS-003''') is a [[Synthetic cannabinoids|synthetic cannabinoid]] that acts as a potent [[cannabinoid]] [[agonist]]. It is highly selective for the [[cannabinoid receptor type 2|cannabinoid-2 receptor]] (CB<sub>2</sub> receptor) subtype, with a selectivity more than 5,000 times greater for the CB<sub>2</sub> receptor than the [[CB1 receptor|CB<sub>1</sub> receptor]].<ref name="Mechoulam 1990">{{cite journal| vauthors = Mechoulam R, Lander N, Breuer A, Zahalka J |date=1990-04-11|title=Synthesis of the individual, pharmacologically distinct enantiomers of a tetrahydrocannabinol derivative|journal=Tetrahedron Asymmetry|volume=1|issue=5|language=en|pages=315–318|doi=10.1016/S0957-4166(00)86322-3|pmid=|pmc=|issn=|doi-access=free}}</ref><ref name="Hanus et al 1999">{{cite journal | vauthors = Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, Pertwee RG, Ross RA, Mechoulam R, Fride E | display-authors = 6 | title = HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 96 | issue = 25 | pages = 14228–14233 | date = December 1999 | pmid = 10588688 | pmc = 24419 | doi = 10.1073/pnas.96.25.14228 | doi-access = free | bibcode = 1999PNAS...9614228H }}</ref><ref>{{cite web |title=Properties of HU-308 ~ Formula C27H42O3 |url=http://pqr.pitt.edu/mol/CFMRIVODIXTERW-BHIFYINESA-N |website=Pitt Quantum Repository |publisher=University of Pittsburgh Department of Chemistry}}</ref> The [[chemical synthesis|synthesis]] and characterization of onternabez took place in the laboratory of [[Raphael Mechoulam]] at the [[Hebrew University of Jerusalem]] (the HU in HU-308) in the late 1990s. The [[pinene]] dimethoxy-DMH-CBD derivative onternabez was identified as a potent peripheral CB<sub>2</sub>-selective agonist in ''in vitro'' and animal studies in 1990<ref name="Mechoulam 1990" /> and 1999.<ref name="Hanus et al 1999" /> |
||
</ref><ref>{{cite web |title=Properties of HU-308 ~ Formula C27H42O3 |url=http://pqr.pitt.edu/mol/CFMRIVODIXTERW-BHIFYINESA-N |website=Pitt Quantum Repository |publisher=University of Pittsburgh Department of Chemistry}}</ref> The [[chemical synthesis|synthesis]] and characterization took place in the laboratory of Prof. [[Raphael Mechoulam|Mechoulam]] at the [[Hebrew University of Jerusalem]], (the HU in HU-308), in the late 1990s. The [[pinene]] dimethoxy-DMH-CBD derivative HU-308 was identified decades ago as a potent peripheral CB<sub>2</sub>-selective agonist in Mechoulam et al. 1990,<ref name="Mechoulam 1990" /> and in Hanus et al. 1999.<ref name="Hanus et al 1999" /> HU-308 has shown very interesting properties such as anti-inflammatory, [[analgesic]], neuroprotective, antitumor and anti-[[Osteoporosis|osteoporitic]] (anti-bone-loss) effects, and has been used as a pharmacological tool in numerous cannabinoid studies contributing to the progress in this field (e.g., Hanus et al. 1999;<ref name="Hanus et al 1999" /> Ofek et al. 2006;<ref name="Ofek et al 2006">{{cite journal| vauthors = Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, Mechoulam R, Zimmer A, Bab I|date=2006-01-17|title=Peripheral cannabinoid receptor, CB<sub>2</sub>, regulates bone mass|journal=Proc Natl Acad Sci U S A|volume=103|issue=3|language=en|pages=696–701|doi=10.1073/pnas.0504187103|pmid=16407142|pmc=1334629|issn=|doi-access=free}}</ref> Rajesh et al. 2007a,<ref name="Rafesh 2007a">{{cite journal| vauthors = Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Huffman JW, et al |date=2007-10-01|title=CB<sub>2</sub>-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion|journal=American Journal of Physiology: Heart & Circulatory Physiology|volume=293|issue=4|language=en|pages=H2210–H2218|doi=10.1152/ajpheart.00688.2007|pmid=17660390|pmc=2229632|issn=|doi-access=free}}</ref> Morales 2017<ref name="Morales">{{cite journal| vauthors = Morales P, Reggio PH, Jagerovic N |date=2017-06-28|title=An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol|journal=Frontiers in Pharmacology|volume=8|issue=|language=en|pages=422|doi=10.3389/fphar.2017.00422|pmid=28701957|pmc=5487438|issn=|doi-access=free}}</ref>), including being named a pivotal advance in the NIH database in Rafesh 2007b for its discoveries, findings and results in attenuating [[oxidative stress]], [[inflammatory response]], and [[apoptosis]] (programmed cell death).<ref name="Rajesh 2007b">{{cite journal | vauthors = Rajesh M, Pan H, Mukhopadhyay P, Bátkai S, Osei-Hyiaman D, Haskó G, Liaudet L, Gao B, Pacher P | display-authors = 6 | title = Pivotal Advance: Cannabinoid-2 receptor agonist HU-308 protects against hepatic Ischemia-reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis | journal = Journal of Leukocyte Biology | volume = 82 | issue = 6 | pages = 1382–9 | date = December 2007 | pmid = 17652447 | pmc = 2225476 | doi = 10.1189/jlb.0307180 }}</ref> HU-308 is also classified as a non-steroidal anti-inflammatory drug (NSAID).<ref name="ES2784229T3 Patent">{{cite web |last1=Lynch |first1=Mary |last2=Kelly |first2=Melanie |title=Spanish Patent ES2784229T3 HU-308, HU-433, CBD-DMH Compositions and procedures for the treatment of eye inflammation and pain |url=https://patents.google.com/patent/ES2784229T3/en?oq=ES2784229T3 |website=Google Patents |publisher=Panag Pharma |access-date=22 February 2021 |quote=A61P29/00 Non-central analgesic, antipyretic or anti-inflammatory agents, e.g antirheumatic agents; Non-steroidal anti-inflammatory drugs (NSAIDs)}}</ref> |
|||
== |
==Legal status== |
||
===Osteoporosis=== |
|||
[[Cannabinoid receptors]] were first implicated in the regulation of [[bone mass]] by Karsak et al. (2004),<ref>{{cite journal| vauthors = Karsak M, Ofek O, Fogel M, Wright K, Tam J, Gabet Y, Birenboim R, Attar-Namdar M, Müller R, Cohen-Solal M |date=October 2004|title=The cannabinoid CB<sub>2</sub> receptor: a potential target for the treatment of osteoporosis|journal=Journal of Bone and Mineral Research|volume=19|issue=S1|language=en|pages=S383|doi=10.1002/jbmr.5650191306|pmid=|pmc=|issn=|doi-access=free}}</ref> who found that CB<sub>2</sub> [[knockout mice]] had markedly accelerated age-related [[Trabecular bone score|trabecular]] bone loss and [[cortex (anatomy)|cortical]] expansion accompanied by increased activity of trabecular [[osteoblast]]s, increased numbers of [[osteoclasts]], and decreased numbers of diaphyseal osteoblast precursors (Ofek et al. 2006).<ref name="Ofek et al 2006" /> CB<sub>2</sub> receptors were expressed in osteoblasts, [[osteocytes]], and osteoclasts. The selective CB<sub>2</sub> agonist HU-308, but not the [[CB1 receptor|CB]]<sub>1</sub> receptor agonist [[noladin ether]], attenuated [[ovariectomy]]-induced bone loss and markedly stimulated cortical thickness through the suppression of osteoclast number and stimulation of endocortical bone formation.<ref name="Ofek et al 2006" /> Furthermore, HU-308 dose dependently increased the number and activity of endocortical osteoblasts and restrained trabecular osteoclastogenesis by inhibiting proliferation of osteoclast precursors.<ref name="Ofek et al 2006" /> These results, coupled with CB<sub>2</sub> but not [[CB1 receptor|CB]]<sub>1</sub> receptor [[mRNA]] expression during osteoblastic differentiation, suggested a role for CB<sub>2</sub> receptors in [[bone remodeling]]. Such a role of CB<sub>2</sub> but not [[CB1 receptor|CB]]<sub>1</sub> receptors is also supported by a systematic [[Genetics|genetic]] association study by Karsak et al. (2005) in human samples of [[postmenopausal osteoporosis]] patients and matched female control subjects, which found a very statistically significant association of single [[Gene polymorphism|polymorphism]]s (P=0.0014) and [[haplotype]]s (P=0.0001) that encompassed the CNR<sub>2</sub> gene on human chromosome 1p36, while finding no convincing association for the [[psychotropic]] CNR<sub>1</sub> gene.<ref name="Karsak et al 2005">{{cite journal| vauthors = Karsak M, Cohen-Solal M, Freudenberg J, Ostertag A, Morieux C, Kornak U, Essig J, Erxlebe E, Bab I, Kubisch C, de Vernejoul MC, Zimmer A |date=15 November 2005|title=Cannabinoid receptor type 2 gene is associated with human osteoporosis|journal=Human [[Molecular Genetics]]|volume=14|issue=22|language=en|pages=3389–96|doi=10.1093/hmg/ddi370|pmid=16204352|pmc=|issn=|doi-access=free}}</ref> It is the non-psychotropic cannabinoid receptor type 2 gene that is found to be so strongly associated with human osteoporosis as P value of 0.0001.<ref name="Karsak et al 2005" /> |
|||
Onternabez is non-psychoactive and not scheduled at the federal level in the United States.<ref>{{Cite web |url=http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm |title=21 CFR — Schedules of controlled substances §1308.11 Schedule I. |access-date=2014-12-17 |archive-date=2009-08-27 |archive-url=https://web.archive.org/web/20090827043725/http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm |url-status=dead }}</ref> It is a Schedule I [[controlled substance]] in the state of [[Florida]] making it illegal to buy, sell, or possess there.<ref>{{cite web | url = http://leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0800-0899/0893/0893.html | work = Florida Statutes | title = Chapter 893 - Drug abuse prevention and control }}</ref> |
|||
===NeuroInflammation=== |
|||
HU308 promotes neural [[Progenitor cell|progenitor]] (NP) [[cell proliferation|proliferation]] and [[neurogenesis]] of [[neural stem cells]],<ref>{{cite journal | vauthors = Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzmán M, Galve-Roperh I | title = Non-psychoactive CB<sub>2</sub> cannabinoid agonists stimulate neural progenitor proliferation | journal = FASEB Journal | volume = 20 | issue = 13 | pages = 2405–7 | date = November 2006 | pmid = 17015409 | doi = 10.1096/fj.06-6164fje | s2cid = 4885167 | url = https://semanticscholar.org/paper/96b76549d320c082222bc4869d612834b1bfcfd9 }} |
|||
</ref> promotes [[neuroprotection]] and neurorepair, activates [[Phosphatidylinositol|Phosphatidyl Inositol]], and has important implications for neuronal survival under [[Neuroinflammation|neuroinflammatory]] conditions occurring in [[animal model]]s of [[neurodegenerative disease]]s, such as [[multiple sclerosis]], [[Alzheimer disease]], and [[Huntington's disease|Huntington's Disease]],<ref>{{cite journal| vauthors = Fernández-Ruiz J, González S, Romero J, Ramos JA |date=2005|title=Cannabinoids in Neurodegeneration and Neuroprotection. |journal=In: Mechoulam, R.(Ed.), Cannabinoids as Therapeutics (MDT) Birkhaüser Verlag; Switzerland|volume=|issue=|language=en|pages=79–109|issn=|doi-access=}}</ref><ref>{{cite journal| vauthors = Fernández-Ruiz J, Romero J, Velasco G, Tolón RM, Ramos JA, Guzmán M |date=Jan 2007|title=Cannabinoid CB<sub>2</sub> receptor: a new target for the control of neural cell survival|journal=Trends Pharmacol Sci|volume=28|issue=1|language=en|pages=:39–45|doi=10.1016/j.tips.2006.11.001|pmid=17141334|pmc=|issn=|doi-access=free}}</ref><ref>{{cite journal| vauthors = Esposito G, Scuderi C, Savani C, Steardo L, Jr, De Filippis D, Cottone P, Iuvone T, Cuomo V, Steardo L |date=Aug 2007|title=Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression|journal=British Journal of Pharmacology|volume=151|issue=8|language=en|pages=1272–1279|doi=10.1038/sj.bjp.0707337|pmid=17592514|pmc=2189818|issn=|doi-access=free}}</ref><ref>{{cite journal| vauthors = Fernández-López D, Pazos MR, Tolón RM, Moro MA, Romero J, Lizasoain I, Martínez-Orgado J |date=Sep 2007|title=The cannabinoid agonist WIN55212 reduces brain damage in an in vivo model of hypoxic-ischemic encephalopathy in newborn rats|journal=Pediatric Research|volume=62|issue=3|language=en|pages=255–60|doi=10.1203/PDR.0b013e318123fbb8|pmid=17622949|pmc=|issn=|doi-access=free}}</ref> and upon acute ischemic [[brain injury]].<ref>{{cite journal | vauthors = Palazuelos J, Ortega Z, Díaz-Alonso J, Guzmán M, and Galve-Roperh I | title = CB<sub>2</sub> Cannabinoid Receptors Promote Neural Progenitor Cell Proliferation via mTORC1 Signaling | journal = Journal of Biological Chemistry | volume = 287 | issue = 2 | pages = 1198–1209 | date = January 2012 | pmid = 22102284 | doi = 10.1074/jbc.M111.291294 | doi-access = free }}</ref> [[Attenuation]] of the inflammatory response in the brain has also been reported by activation of CB<sub>2</sub> receptors in a study of [[Pia mater|pial]] vessels forming the blood–brain barrier, using a model of [[lipopolysaccharide]]-induced encephalitis (Ramirez et al. 2012), wherein activation of CB<sub>2</sub> receptors decreased [[adhesion molecules]] in the brain tissue and [[Leukocyte adhesion cascade|leukocyte-endothelial adhesion]] in the pial vessels.<ref>{{cite journal| vauthors = Ramirez SH, Haskó J, Skuba A, Fan S, Dykstra H, McCormick R, Reichenbach N, Krizbai I, Mahadevan A, Zhang M, Tuma R, Son YJ, Persidsky Y |date=21 March 2012|title=Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions|journal=J Neuroscience|volume=32|issue=12|language=en|pages=4000–16|doi=10.1523/JNEUROSCI.4628-11.2012|pmid=22442067|pmc=3325902|issn=|doi-access=free}}</ref> HU-308 protects both [[liver]] and [[blood vessel]] tissues against hepatic ischemia and reperfusion ([[blood circulatory system]]) injury by attenuating [[oxidative stress]], [[inflammatory response]] and [[apoptosis]] via inhibition of [[TNF-α]].<ref name="Rafesh 2007a" /> The role of CB<sub>2</sub> receptors in endothelial cell activation and endothelial/inflammatory cell interactions, being critical steps not only in [[reperfusion injury]], but also [[atherosclerosis]] and other inflammatory disorders, turned out to be very important, because selective CB<sub>2</sub> cannabinoid agonist HU-308 decreased TNF-α-induced [[ICAM-1]] and [[VCAM-1]] expression in human liver [[sinusoidal endothelial cells]] (HLSECs) expressing CB<sub>2</sub> receptors, as well as the adhesion of human [[neutrophils]] to HLSECs in vitro.<ref>{{cite journal| vauthors = Pacher P, Gao B |date=Apr 2008|title=Endocannabinoids and Liver Disease. III. Endocannabinoid effects on immune cells: implications for inflammatory liver diseases|journal=Am J Physiol Gastrointest Liver Physiol|volume=294|issue=4|language=en|pages=G850–G854|doi=10.1152/ajpgi.00523.2007|pmid=18239059|pmc=2376822|issn=|doi-access=free}}</ref> HU-308 reduces blood pressure, blocks defecation, and elicits anti-inflammatory and peripheral analgesic activity.<ref name="Hanus et al 1999" /><ref>{{cite journal | vauthors = LaBuda CJ, Koblish M, Little PJ | title = Cannabinoid CB<sub>2</sub> receptor agonist activity in the hindpaw incision model of postoperative pain | journal = European Journal of Pharmacology | volume = 527 | issue = 1–3 | pages = 172–4 | date = December 2005 | pmid = 16316653 | doi = 10.1016/j.ejphar.2005.10.020 }}</ref> Currently, CBD (especially potent CBD derivatives like HU-308) generate considerable interest due to their beneficial neuroprotective, antiepileptic, anxiolytic, antipsychotic, anti-inflammatory and pain-relieving properties, therefore, the CBD scaffold becomes of increasing interest for medicinal chemists.<ref name="Morales" /> |
|||
== See also == |
|||
===Inflammation & Immune Modulation=== |
|||
* [[Cannabidiol dimethyl ether]] |
|||
HU-308 has an important functional outcome ~ the secretion of [[interleukin 6]] (IL-6) and [[interleukin 10]] (IL-10) with therapeutic [[immunomodulatory]] properties in ''vitro.''<ref>{{cite journal| vauthors = Saroz Y, Kho DT, Glass M, Graham ES, Grimsey NL |date=2019-10-19|title=Cannabinoid Receptor 2 (CB 2 ) Signals via G-alpha-s and Induces IL-6 and IL-10 Cytokine Secretion in Human Primary Leukocytes|journal=ACS Pharmacology & Translational Science|volume=2|issue=6|language=en|pages=414–428|doi=10.1021/acsptsci.9b00049|pmid=32259074|pmc=7088898|issn=2575-9108|doi-access=free}}</ref> There is evidence that IL-6 may be used as an inflammatory marker for the more severe COVID-19 infections that have a poor prognosis for a favorable outcome because raised levels of IL-6 as well as troponin are associated with a poor prognosis in COVID-19.<ref>{{cite web |title=Raised troponin and interleukin-6 levels are associated with a poor prognosis in COVID-19. 2 April 2020. Graham Cole (Imperial College Healthcare NHS Trust, London, UK) |url=https://cardiacrhythmnews.com/raised-troponin-and-interleukin-6-levels-are-associated-with-a-poor-prognosis-in-covid-19/ |website=Cardiac Rhythm News |publisher=Graham Cole, CRN |access-date=21 February 2021}}</ref> Researchers Dr. Melanie Kelly and Dr. C. Lehmann at Panag Pharma, now merged with Tetra Bio-Pharma,<ref>[https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2018/Tetra-Bio-Pharma-Enters-into-Non-Binding-Proposal-to-Acquire-Panag-Pharma-Inc/default.aspx Nov 2018, Tetra Bio-Pharma Enters into Non-Binding Proposal to Acquire Panag Pharma Inc.]</ref><ref>[https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2019/Tetra-Bio-Pharma-Enters-into-Definitive-Agreement-to-Acquire-Panag-Pharma-Inc/default.aspx Jan 2019, Tetra Bio-Pharma Enters into Definitive Agreement to Acquire Panag Pharma Inc.]</ref><ref>[https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2019/Tetra-Bio-Pharma-Shareholders-Approve-the-Acquisition-of-Panag-Pharma/default.aspx Apr 2019, Tetra Bio-Pharma Shareholders Approve the Acquisition of Panag Pharma]</ref><ref>[https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2019/Tetra-Bio-Pharma-Closesthe-Acquisition-of-Panag-Pharma/default.aspx May 2019, Tetra Bio-Pharma Closes the Acquisition of Panag Pharma]</ref> which owns the IP rights to HU-308,<ref>[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=14722991&OS=14722991&RS=14722991 USPTO], Compositions and methods for treatment of ocular inflammation and/or pain (Lynch & Kelly Jan 2017). In certain embodiments, the non-psychotropic phytocannabinoid is beta-caryophyllene or cannabidiol [CBD] and the synthetic cannabinoid is HU-433, HU-308, or a modified CBD such as CBD-DMH.</ref><ref>[https://patents.justia.com/patent/9549906#citations Justia], Compositions and methods for treatment of ocular inflammation and/or pain (Lynch & Kelly May 2015)</ref><ref>{{cite web |last1=Lynch |first1=Mary |last2=Kelly |first2=Melanie |title=Patent 9549906 Composition & Methods for Treatment of Ocular Inflammation &/or Pain Jan 2017 |url=https://assignment.uspto.gov/patent/index.html#/patent/search/resultAbstract?id=9549906&type=patNum |website=U.S. Patent & Trademark Office |publisher=USPTO, Panag Pharma |access-date=20 February 2021}}</ref> showed with Drs. J Sardinha and J Zhou that HU 308 also mediates [[immune modulation]] in sepsis,<ref>{{cite journal | vauthors = Sardinha, Kelly, Zhou, Lehmann | title = Experimental cannabinoid 2 receptor-mediated immune modulation in sepsis | journal = Mediators of Inflammation | date = 2014 | pmid = 24803745 }}</ref> as well as displays [[antiallodynic]] activity (alleviates [[Allodynia|allodynic]] pain) in the rat hindpaw incision model of post-operative pain, is [[neuroprotective]] and improves [[Motor skill|motor performance]] in a [[Model organism|mouse model]] of [[Huntington's Disease]].<ref>https://www.tocris.com/products/hu-308_3088</ref> Continued work by Dr. MEM Kelly et al. showed HU-308 also dramatically fights the [[Cytokine release syndrome|Cytokine Release Syndrome]] (CRS), also called cytokine release storm, that is seen in many diseases and conditions, including [[Acute Respiratory Distress Syndrome]] (ARDS), [[COVID-19]], [[Sepsis]], [[Septic Shock]], [[Systemic inflammatory response syndrome|Systemic Inflammatory Response Syndrome]] (SIRS), [[Cytokine storm|Cytokine Storm Syndrome]] (CSS), [[Multiple organ dysfunction syndrome|Multi-Organ Dysfunction Syndrome]] (MODS), [[Pneumonia]], [[Uveitis]], Corneal [[Neuropathic pain|Neuropathic Pain]] [[Hyperalgesia]], [[Allodynia|Photo-allodynia]], Burning, Stinging, Dryness and [[Inflammation]]. The [[antinociceptive]] and [[anti-inflammatory]] effects of HU-308, but not Δ<sup>8</sup>THC or CBD, were mediated through CB<sub>2</sub>R, and it reduces [[cytokine storm]]s in the eye, importantly, where [[corneal]] damage can result in an [[Inflammation|inflammatory response]] that involves the production of proinflammatory cytokines, [[neovascularization]], recruitment of [[leukocytes]], and release of [[neuropeptides]] producing inflammatory pain.<ref>https://www.cas.org/blog/covid-19-cytokine-storms</ref><ref>https://pubmed.ncbi.nlm.nih.gov/31613449/</ref><ref name="Thapa">{{cite journal | vauthors = Thapa, Cairns, Szczesniak, Toguri, Caldwell, Kelly | title = The Cannabinoids Δ8THC, CBD, and HU-308 Act via Distinct Receptors to Reduce Corneal Pain and Inflammation | journal = Cannabis & Cannabinoid Research | date = 2018 | pmid = 29450258}}</ref> The Thapa et al. study on HU-308 in reducing Corneal Pain in 2018 is the first time a CB<sub>2</sub>R agonist has been demonstrated to reduce corneal pain.<ref name="Thapa" /> HU-308 is a selective and highly potent agonist at CB<sub>2</sub>R and has previously been shown to reduce [[lipopolysaccharide]]-induced intraocular inflammation.<ref name="Thapa" /><ref>{{cite journal | vauthors = Toguri, Lehmann, Laprairie, Szczesniak, Zhou, Denovan-Wright, Kelly | display-authors = | title = Anti-inflammatory effects of cannabinoid CB(2) receptor activation in endotoxin-induced uveitis | journal = British Journal of Pharmacology | volume = 171 | issue = 6 | pages = 1448–61 | date = March 2014 | pmid = 24308861 | pmc = 3954484 | doi = 10.1111/bph.12545 }}</ref> |
|||
* [[Cannabidiol diacetate]] |
|||
* [[HU-210]] |
|||
===Multi-Organ Dysfunction & Damage=== |
|||
* [[HU-320]] |
|||
While CB<sub>2</sub> [[knockout mice]] developed enhanced inflammation and tissue injury from [[cisplatin]]-induced [[kidney damage]], HU-308, working through the [[endocannabinoid system]] and the CB<sub>2</sub> receptor, protected against cisplatin-induced kidney damage by attenuating inflammation and [[Oxidative stress|oxidative]] or [[nitrosative stress]], and such selective CB<sub>2</sub> agonists may represent a promising novel approach to prevent this devastating complication of [[chemotherapy]].<ref name="Mukhopadhyay">{{cite journal | vauthors = Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Bátkai S, Gao B, Haskó G, Pacher P | display-authors = 7 | title = Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress and cell death in nephropathy | journal = Free Radical Biology and Medicine | volume = 48 | issue = 3 | pages = 457–67 | date = February 2010 | pmid = 19969072 | pmc = 2869084 | doi = 10.1016/j.freeradbiomed.2009.11.022 }}</ref> Activation of the cannabinoid-2 (CB<sub>2</sub>) receptors (expressed predominantly in [[immune cells]], and also to a much less extent in other cell types, e.g., endothelial and [[parenchymal]] cells) by recently recognized endogenous [[lipid]] mediators (the endocannabinoids) produced and present in virtually all tissues/organ systems,<ref>{{cite journal | vauthors = Mechoulam R, Fride E, DiMarzo V | display-authors = | title = Endocannabinoids. | journal = Eur J Pharmacol. | volume = 359 | issue = 1 | pages = 1–18 | date = 1998 | pmid = 9831287 | pmc = | doi = 10.1016/s0014-2999(98)00649-9 }}</ref><ref>{{cite journal | vauthors = Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG | display-authors = | title = International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. | journal = Pharmacol Rev | volume = 54 | issue = 2 | pages = 161–202 | date = 2002 | pmid = 12037135 | pmc = | doi = 10.1124/pr.54.2.161 }}</ref><ref>{{cite journal | vauthors = Pacher P, Batkai S, Kunos G | display-authors = | title = International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. The Endocannabinoid System as an Emerging Target of Pharmacotherapy | journal = Pharmacol Rev | volume = 58 | issue = 3 | pages = 389–462 | date = Sep 2006 | pmid = 16968947 | pmc = 2241751 | doi = 10.1124/pr.58.3.2 }}</ref> or by selective synthetic CB<sub>2</sub> agonists such as HU-308 in the pivotal advance by Rajesh et al. (2007),<ref name="Rajesh 2007b" /> has been shown to protect against tissue damage in various experimental models of ischemic-reperfusion injury,<ref name="Rajesh 2007b" /><ref>{{cite journal| vauthors = Batkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, Hong F, Harvey-White J, Jafri A, Hasko G, Huffman JW, Gao B, Kunos G, Pacher P |date=Jun 2007|title=Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury|journal=FASEB J|volume=21|issue=8|language=en|pages=1788–1800|doi=10.1096/fj.06-7451com|pmid=17327359|pmc=2228252|issn=|doi-access=free}}</ref> atherosclerosis/cardiovascular inflammation,<ref>{{cite journal| vauthors = Gallily R, Breuer A, Mechoulam R |date=2000-10-06|title=2-Arachidonylglycerol, an endogenous cannabinoid, inhibits tumor necrosis factor-alpha production in murine macrophages, and in mice|journal=Eur J Pharmacol|volume=406|issue=1|language=en|pages=R5-7|doi=10.1016/s0014-2999(00)00653-1|pmid=11011050|pmc=|doi-access=free}}</ref><ref>{{cite journal| vauthors = Gaoni Y, Mechoulam R |date=1971-01-13|title=The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish|journal=J Am Chem Soc|volume=93|issue=1|language=en|pages=217–24|doi=10.1021/ja00730a036|pmid=5538858|pmc=|issn=|doi-access=free}}</ref><ref>{{cite journal| vauthors = García-Arencibia M, González S, de Lago E, Ramos JA, Mechoulam R, Fernández-Ruiz J |date=2007-02-23|title=Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: importance of antioxidant and cannabinoid receptor-independent properties|journal=Brain Res|volume=1134|issue=1|language=en|pages=162–70|doi=10.1016/j.brainres.2006.11.063|pmid=17196181|pmc=|issn=|doi-access=free}}</ref> and neurodegenerative,<ref>{{cite journal| vauthors = Sagredo O, González S, Aroyo I, Pazos M, Benito C, Lastres-Becker I, Romero J, Tolón R, Mechoulam R, Brouillet E, Romero J, Fernández-Ruiz J |date=2009-08-15|title=Cannabinoid CB<sub>2</sub> receptor agonists protect the striatum against malonate toxicity: Relevance for Huntington’s disease|journal=Glia|volume=57|issue=11|language=en|pages=1154–67|doi=10.1002/glia.20838|pmid= |pmc=2706932 }}</ref> [[gastrointestinal]]<ref name="Ke 2016">{{cite journal| vauthors = Ke P, Shao BZ, Xu ZQ, et al |date=2016-09-09|title=Activation of Cannabinoid Receptor 2 Ameliorates DSS-Induced Colitis through Inhibiting NLRP3 Inflammasome in Macrophages|journal=PLoS One|volume=11|issue=9|language=en|pages=e0155076|doi=10.1371/journal.pone.0155076|pmid=27611972|pmc=5017608|issn=|doi-access=free}}</ref><ref>{{cite journal| vauthors = Storr MA, Keenan CM, Zhang H, Patel KD, Makriyannis A, Sharkey KA |date=Nov 2009|title=Activation of the cannabinoid 2 receptor (CB(2)) protects against experimental colitis|journal=Inflammable Bowel Disease|volume=15|issue=11|language=en|pages=1678–1685|doi=10.1002/ibd.20960|pmid=19408320|pmc=5531765|issn=|doi-access=free}}</ref> and other disorders by limiting inflammatory cell [[chemotaxis]]/[[Infiltration (medical)|infiltration]], activation and interrelated oxidative/nitrosative stress.<ref>{{cite journal| vauthors = Pacher P, Batkai S, Kunos G |date=Sep 2006|title=The endocannabinoid system as an emerging target of pharmacotherapy|journal=Pharmacol Rev.|volume=58|issue=3|language=en|pages=389–462|doi=10.1124/pr.58.3.2|pmid=16968947|pmc=2241751|issn=|doi-access=free}}</ref><ref>{{cite journal| vauthors = Bátkai S, Járai Z, Wagner JA, Goparaju SK, Varga K, Liu J, Wang L, Mirshahi F, Khanolkar AD, Makriyannis A, Urbaschek R, Garcia N Jr, Sanyal AJ, Kunos G |date=July 2001|title=Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis|journal=Nat Med|volume=7|issue=7|language=en|pages=827–32|doi=10.1038/89953|pmid=11433348|pmc=|issn=|doi-access=free}}</ref><ref>{{cite journal| vauthors = Engeli S, Böhnke J, Feldpausch M, Gorzelniak K, Janke J, Bátkai S, Pacher P, Harvey-White J, Luft FC, Sharma AM, Jordan J |date=October 2005|title=Activation of the Peripheral Endocannabinoid System in Human Obesity|journal=Diabetes|volume=54|issue=10|language=en|pages=2838–2843|doi=10.2337/diabetes.54.10.2838|pmid=16186383|pmc=2228268|issn=|doi-access=free}}</ref><ref>{{cite journal| vauthors = Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L |date=May 2005|title=Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity|journal=J Clin Invest|volume=115|issue=5|language=en|pages=1298–305|doi=10.1172/JCI23057|pmid=15864349|pmc=1087161|issn=|doi-access=free}}</ref> In vivo, HU308 treatment attenuated DSS-induced [[colitis]] mice associated with reduced colon inflammation and inhibited [[NLRP3]] [[inflammasome]] activation in wild-type mice.<ref name="Ke 2016" /> Furthermore, CB<sub>2</sub> receptors are over-expressed in a variety of cancers, and CB<sub>2</sub> activation may decrease the proliferation and growth of various [[cancer cells]] and [[tumors]].<ref name="Mukhopadhyay" /><ref>{{cite journal| vauthors = Kunikowska G, Jenner P |date=2001-12-13|title=6-Hydroxydopamine-lesioning of the nigrostriatal pathway in rats alters basal ganglia mRNA for copper, zinc- and manganese-superoxide dismutase, but not glutathione peroxidase|journal=Brain Res|volume=922|issue=1|language=en|pages=51–64|doi=10.1016/s0006-8993(01)03149-3|pmid=11730701|pmc=|issn=|doi-access=free}}</ref> HU-308 was shown to reduce swelling, [[Synovial joint|synovial join inflammation and destruction]], in addition to lowering circulating [[antibodies]] against [[Collagen I]].<ref>{{cite journal| vauthors = Gui H, Liu X, Liu LR, Su DF, Dai SM |date=Jun 2015|title=Activation of cannabinoid receptor 2 attenuates synovitis and joint distruction in collagen-induced arthritis|journal=Immunobiology|volume=220|issue=6|language=en|pages=817–22|doi=10.1016/j.imbio.2014.12.012|pmid=25601571|pmc=|issn=|doi-access=free}}</ref> |
|||
* [[HU-211]] |
|||
* [[Nabilone]] |
|||
===ARDS-003=== |
|||
* [[CP 47,497]] |
|||
HU-308, aka ARDS-03 for its ARDS fighting abilities, is currently in a collaboration study by Tetra Bio-Pharma, Targeted Pharmaceutical, LLC, [[George Mason University]] and the [[NIH]] at the university's top-level National Center for Biodefense and Infectious Diseases Biomedical Research Laboratory (BRL) against the lethal condition [[Acute Respiratory Distress Syndrome]] (ARDS) seen in [[COVID-19]] patients.<ref name="forbes1">https://www.forbes.com/sites/emilyearlenbaugh/2020/08/20/synthetic-cannabinoid-drug-for-covid-19-approved-for-phase-1-clinical-trials/</ref><ref>https://s24.q4cdn.com/136309390/files/doc_presentation/2020/12/Tetra-Bio-Pharma-Milestones-Update-Dec.-30-2020.pdf</ref><ref>https://www.sedar.com/GetFile.do?lang=EN&docClass=7&issuerNo=00026458&issuerType=03&projectNo=03122925&docId=4813488</ref><ref>https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2020/Tetra-Bio-Pharma-Targeted-Pharmaceutical--the-George-Mason-University-Partner-on-ARDS-003-to-Prevent--Treat-COVID-19/default.aspx</ref><ref name="Liotta">https://science.gmu.edu/directory/lance-liotta</ref><ref>https://tetrabiopharma.com/partners/</ref> Regulatory filings show that in late 2020 Tetra and Targeted designed short- to mid- term studies to gather additional data on the benefits of ARDS-003 in Sars-CoV-2 infected animal models for the prevention of ARDS in COVID-19.<ref name="SEDAR-17-2-2021">{{cite web |title=SEDAR TBP Annual Report 17 Feb 2021 |url=https://www.sedar.com/GetFile.do?lang=EN&docClass=7&issuerNo=00026458&issuerType=03&projectNo=03174069&docId=4888388&fbclid=IwAR3W82_NAVXYU0QVVyDBfWrZi7qW7KMBol6cweldmB3GXNTlggMrANeKdbo |website=SEDAR |publisher=SEDAR Tetra Bio-Pharma |access-date=20 February 2021}}</ref> A former Deputy Director of the NIH is heading the GMU research on ARDS-003, which is a novel, sterile, injectable, optimized, [[nanoemulsion]] form of HU-308 that has successfully undergone stringent safety and [[toxicology]] studies in accordance with U.S. FDA oversight, which were required before submitting an [[investigational new drug]] (IND) application in the US and a clinical trial application (CTA) in Canada for a [[Phase 1 clinical testing|Phase 1 research study]] through the Sars-CoV-2 regulatory fast track pathway.<ref name="Liotta" /><ref name="TBP ARDS3 News">https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2020/Tetra-Bio-Pharma-Completes-Major-Milestone-for-COVID-19-Therapeutic/default.aspx</ref> The toxicology program was designed to the standards of the [[International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use|International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use]] (ICH) for enabling a [[First-in-human trial|first-in-human]] clinical trial; and included general toxicology data for two species-specific studies to assess toxicity in major organ systems ([[cardiovascular]], [[respiratory]], [[nervous system]]) and [[genotoxicity]], as well as the [[metabolism]] and [[Pharmacokinetics|pharmacokinetic]] distribution of the drug.<ref name="TBP ARDS3 News" /> Tetra Bio-Pharma is the first [[endocannabinoid system]] (ECS) biotechnology company researching a cannabinoid treatment for ARDS and sepsis linked to COVID-19, pneumonia and other critical conditions, and the ARDS-003 pharmaceutical drug now has FDA approval to begin Phase I and Phase II clinical trials in human subjects for the reduction of cytokine storm, sepsis, and ARDS in COVID-19.<ref name="forbes1" /><ref name="TBP ARDS3 News" /> [[George Mason University Notable Faculty and Alumni|GMU]] researchers including the Co-Director, Center for Applied [[Proteomics]] and [[Molecular medicine|Molecular Medicine]] (CAPMM), are conducting three studies to assess the therapeutic efficacy of candidate interventions for COVID-19 in mouse models of [[Angiotensin-converting enzyme 2 |angiotensin-converting enzyme 2 (ACE2)]] animals infected [[Nasal administration|intranasally]] with [[SARS-CoV-2]] to determine the survival advantage conferred by a therapeutic, to determine the survival advantage conferred by a therapeutic if an alternate course or dosing strategy needs to be followed, and to determine viral levels on day three post-infection when [[viral load]] in the [[lung]]s is expected to peak.<ref>{{cite web |last1=Narayanan |first1=Aarthi |last2=Liotta |first2=Lance |title=GMU Grant Announcement: Narayanan and Liotta testing therapeutic efficacy of potential COVID-19 treatments |url=https://www.eurekalert.org/pub_releases/2021-02/gmu-nl021921.php |website=EurekAlert! operated by the nonprofit American Association for the Advancement of Science (AAAS) |publisher=George Mason University |access-date=24 February 2021}}</ref> Dalton Pharma is producing the injectable drug for the GMU effort.<ref>{{cite web |last1=Cachapero |first1=Joanne |title=Cannabis and Coronavirus: Sales Surge as the Industry Carries On |url=https://mgretailer.com/cannabis-news/cannabis-and-coronavirus-sales-surge-as-the-industry-carries-on/ |website=MG Retailer |access-date=28 February 2021 |quote=Canadian cannabis pharmaceutical company Tetra Bio-Pharma recently contracted with Dalton Pharma Services to produce batches of its HU-308 and ARDS-003, which could help to treat severe cytokine reactions.}}</ref> |
|||
===Ocular Diseases=== |
|||
A 50-50 [[joint venture]] collaboration of Tetra Bio-Pharma and Altus Formulations, named TALLC, is developing a fast onset, a long-duration form of HU-308, called TA-A001, which is a non-opioid, non-steroidal [[small molecule]] of low molecular weight: (< 900 [[Dalton (unit)|daltons]]) that selectively activates CB2 receptors mediating the human anti-inflammatory response, as an alternative to opioid medications and NSAIDs for ocular pain and [[Dry eye syndrome|dry eye]] disease.<ref name="TBP 2020 Annual Report">{{cite web |title=Tetra Bio-Pharma 2020 Annual Report filed with SEDAR |url=https://www.sedar.com/GetFile.do?lang=EN&docClass=7&issuerNo=00026458&issuerType=03&projectNo=03174069&docId=4888388 |website=SEDAR |publisher=Tetra Bio-Pharma |access-date=24 February 2021}}</ref> TA-A001 uses the JV's patented SmartCelle nanotechnology, and a second formulation, called TA-P2005, is shown in preclinical studies to have twelve times more efficacy than if conventionally formulated and is expected to increase [[cornea]]l access to the medication.<ref name="TBP 2020 Annual Report" /> Ongoing studies seek to determine analgesic and anti-inflammatory effects of various dosages of TA-A001 in a corneal [[Hyperalgesia|hyperalgesia model]].<ref name="TBP 2020 Annual Report" /> TA-A002 is a further topical treatment for treating the [[glaucoma]] and dry-eye disease.<ref name="TBP 2020 Annual Report" /> TALLC's lead indication is the [[orphan disease]] [[keratoconus]], for which it is submitting its application for [[orphan drug]] status.<ref name="TBP 2020 Annual Report" /> On July 7, 2020, the United Kingdom Intellectual Property Office granted the TALLC patent GB2561009 for its novel SmartCelle nanotechnology.<ref name="GB2561009A">{{cite web |last1=Smith |first1=Damon |last2=Baille |first2=Wilms |last3=Kujawa |first3=Piotr |title=Great Britain Patent GB2561009 Non-ionic block copolymers and pharmaceutical compositions derived therefrom |url=https://patents.google.com/patent/GB2561009A/en?oq=GB2561009 |website=Google Patents |publisher=Tetra Bio-Pharma, Altus (by 50-50 JV:TALLC) |access-date=23 February 2021 |quote=Abstract: There are provided PVP-PLA block copolymers as defined in Formula I: wherein, x is an initiator alcohol having a boiling point greater than 145°C, n is, on average, from 20 and 40, and m is, on average, from 10 and 40, wherein the block copolymers have a number average molecular weight (Ma) of at least 3000 Da. Polymers demonstrating flexibility in formulating multiple low-solubility active pharmaceutical ingredients (APIs) are described. Liquid and dry pharmaceutical formulations comprising an API are described, along with delivery methods, uses, and kits. APIs may include, e.g. flurbiprofen, celecoxib, acetaminophen, or propofol. Also provided is a method of synthesizing the PVP-PLA block copolymers by (i) initiating polymerization of D, L-Lactide from the initiator alcohol x to form poly(lactic acid), adding a xanthate, e.g Potassium O-Ethyl Xanthate, to form a PLA macroinitiator, and polymerizing NVP onto the PLA macroinitiator, by controlled polymerization, to form the block copolymer compound of Formula I.}}</ref> It is the first patent granted in the series of SmartCelleT “smart [[micelle]]” patents applied for and the TA-A001 reformulation of HU-308 is considered, like HU-308, to also be a [[nonsteroidal anti-inflammatory drug]] (NSAID), which is a drug class that reduces pain and [[fever]], prevents [[blood clots]], and in higher doses decreases inflammation.<ref name="TBP 2020 Annual Report" /> |
|||
==Legal status== |
|||
HU-308 is non-psychoactive and not scheduled at the federal level in the United States.<ref>[http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm 21 CFR — Schedules of controlled substances §1308.11 Schedule I.]</ref> It is a Schedule I [[controlled substance]] in the state of [[Florida]] making it illegal to buy, sell, or possess there.<ref>[http://leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0800-0899/0893/0893.html Florida Statutes - Chapter 893 - Drug abuse prevention and control]</ref> |
|||
== References == |
== References == |
||
{{reflist}} |
{{reflist}} |
||
{{Cannabinoids}} |
{{Cannabinoids}} |
||
== See also == |
|||
* [[HU-210]] |
|||
* [[HU-320]] |
|||
[[Category:Primary alcohols]] |
[[Category:Primary alcohols]] |
||
Latest revision as of 08:24, 16 October 2024
 | |
| Clinical data | |
|---|---|
| Other names | HU-308, HU308, PPP-003, ARDS-003 |
| Drug class | CB2 receptor agonist |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Excretion | Kidneys |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H42O3 |
| Molar mass | 414.630 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Onternabez (also known as HU-308, HU308, PPP-003, and ARDS-003) is a synthetic cannabinoid that acts as a potent cannabinoid agonist. It is highly selective for the cannabinoid-2 receptor (CB2 receptor) subtype, with a selectivity more than 5,000 times greater for the CB2 receptor than the CB1 receptor.[1][2][3] The synthesis and characterization of onternabez took place in the laboratory of Raphael Mechoulam at the Hebrew University of Jerusalem (the HU in HU-308) in the late 1990s. The pinene dimethoxy-DMH-CBD derivative onternabez was identified as a potent peripheral CB2-selective agonist in in vitro and animal studies in 1990[1] and 1999.[2]
Legal status
[edit]Onternabez is non-psychoactive and not scheduled at the federal level in the United States.[4] It is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess there.[5]
See also
[edit]References
[edit]- ^ a b Mechoulam R, Lander N, Breuer A, Zahalka J (1990-04-11). "Synthesis of the individual, pharmacologically distinct enantiomers of a tetrahydrocannabinol derivative". Tetrahedron Asymmetry. 1 (5): 315–318. doi:10.1016/S0957-4166(00)86322-3.
- ^ a b Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. (December 1999). "HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor". Proceedings of the National Academy of Sciences of the United States of America. 96 (25): 14228–14233. Bibcode:1999PNAS...9614228H. doi:10.1073/pnas.96.25.14228. PMC 24419. PMID 10588688.
- ^ "Properties of HU-308 ~ Formula C27H42O3". Pitt Quantum Repository. University of Pittsburgh Department of Chemistry.
- ^ "21 CFR — Schedules of controlled substances §1308.11 Schedule I." Archived from the original on 2009-08-27. Retrieved 2014-12-17.
- ^ "Chapter 893 - Drug abuse prevention and control". Florida Statutes.
