Onternabez: Difference between revisions
ce |
Slothwizard (talk | contribs) No edit summary Tags: Visual edit Mobile edit Mobile web edit |
||
| (41 intermediate revisions by 18 users not shown) | |||
| Line 1: | Line 1: | ||
{{short description| |
{{short description|Cannabidiol-derivative drug}} |
||
{{Copy edit|for=Capitalization, name-dropping of individuals by last name who are not universally known by readers|date=February 2021}} |
|||
{{Drugbox |
{{Drugbox |
||
| Verifiedfields |
| Verifiedfields = changed |
||
| Watchedfields |
| Watchedfields = changed |
||
| class = [[CB2 receptor|CB<sub>2</sub> receptor agonist]] |
|||
| verifiedrevid = 426291380 |
|||
| verifiedrevid = 426291380 |
|||
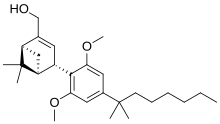
| IUPAC_name = [(1''R'',2''R'',5''R'')-2-[2,6-Dimethoxy-4-(2-methyloctan-2-yl)phenyl]-7,7-dimethyl-4-bicyclo[3.1.1]hept-3-enyl]methanol |
|||
| IUPAC_name = [(1''S'',2''S'',5''S'')-2-[2,6-Dimethoxy-4-(2-methyloctan-2-yl)phenyl]-7,7-dimethyl-4-bicyclo[3.1.1]hept-3-enyl]methanol |
|||
| image = HU-308.png |
|||
| image = Onternabez.svg |
|||
| caption = |
|||
| caption = |
|||
| width = |
|||
| width = <!--Clinical data--> |
|||
| tradename = |
|||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|||
| pregnancy_category = |
|||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> |
|||
| legal_CA = Schedule II |
|||
| legal_UK = Class B |
|||
| legal_US = Unscheduled <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> <BR> |
|||
| legal_US_comment = |
|||
| legal_status = Florida: Schedule I |
|||
| routes_of_administration = <!--Pharmacokinetic data--> |
|||
| bioavailability = |
|||
| protein_bound = |
|||
| metabolism = [[Liver]] |
|||
| elimination_half-life = |
|||
| excretion = Kidneys |
|||
<!--Identifiers-->| CAS_number_Ref = {{cascite|correct|CAS}} |
|||
<!--Clinical data--> |
|||
| CAS_number = 256934-39-1 |
|||
| tradename = |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|||
| UNII = 8I5L034D55 |
|||
| pregnancy_category = |
|||
| ATC_prefix = None |
|||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> |
|||
| ATC_suffix = |
|||
| legal_CA = Schedule II |
|||
| PubChem = 11553430 |
|||
| legal_UK = Class B |
|||
| ChEBI = 146244 |
|||
| legal_US = Unscheduled <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> <BR> |
|||
| synonyms = HU-308, HU308, PPP-003, ARDS-003 |
|||
| legal_US_comment = |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| legal_status = Florida: Schedule I |
|||
| DrugBank = |
|||
| routes_of_administration = injection, oral, eyedrops |
|||
| KEGG = D12305 |
|||
| ChemSpiderID = 8020425 |
|||
<!--Pharmacokinetic data--> |
|||
<!--Chemical data-->| C = 27 |
|||
| bioavailability = |
|||
| H = 42 |
|||
| protein_bound = |
|||
| O = 3 |
|||
| metabolism = [[Liver]] |
|||
| smiles = CCCCCCC(C)(C)C1=CC(=C(C(=C1)OC)[C@H]2C=C([C@@H]3C[C@H]2C3(C)C)CO)OC |
|||
| elimination_half-life = |
|||
| StdInChI = 1S/C27H42O3/c1-8-9-10-11-12-26(2,3)19-14-23(29-6)25(24(15-19)30-7)20-13-18(17-28)21-16-22(20)27(21,4)5/h13-15,20-22,28H,8-12,16-17H2,1-7H3/t20-,21-,22+/m0/s1 |
|||
| excretion = Kidneys |
|||
| StdInChIKey = CFMRIVODIXTERW-FDFHNCONSA-N |
|||
<!--Identifiers--> |
|||
| CAS_number_Ref = {{cascite|changed|CAS}} |
|||
| CAS_number = 256934-39-1 |
|||
| UNII_Ref = {{fdacite|changed|FDA}} |
|||
| UNII = 8I5L034D55 |
|||
| ATC_prefix = |
|||
| ATC_suffix = |
|||
| PubChem = 5311172 |
|||
| ChEBI = 146244 |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| DrugBank = |
|||
| ChemSpiderID = 8020425 |
|||
<!--Chemical data--> |
|||
| C = 27 | H = 42 | O = 3 |
|||
| smiles = CCCCCCC(C)(C)C1=CC(=C(C(=C1)OC)[C@H]2C=C([C@@H]3C[C@H]2C3(C)C)CO)OC |
|||
| StdInChI = 1S/C27H42O3/c1-8-9-10-11-12-26(2,3)19-14-23(29-6)25(24(15-19)30-7)20-13-18(17-28)21-16-22(20)27(21,4)5/h13-15,20-22,28H,8-12,16-17H2,1-7H3/t20-,21-,22+/m0/s1 |
|||
| StdInChIKey = CFMRIVODIXTERW-FDFHNCONSA-N |
|||
}} |
}} |
||
'''HU-308''', |
'''Onternabez''' (also known as '''HU-308''', '''HU308''', '''PPP-003''', and '''ARDS-003''') is a [[Synthetic cannabinoids|synthetic cannabinoid]] that acts as a potent [[cannabinoid]] [[agonist]]. It is highly selective for the [[cannabinoid receptor type 2|cannabinoid-2 receptor]] (CB<sub>2</sub> receptor) subtype, with a selectivity more than 5,000 times greater for the CB<sub>2</sub> receptor than the [[CB1 receptor|CB<sub>1</sub> receptor]].<ref name="Mechoulam 1990">{{cite journal| vauthors = Mechoulam R, Lander N, Breuer A, Zahalka J |date=1990-04-11|title=Synthesis of the individual, pharmacologically distinct enantiomers of a tetrahydrocannabinol derivative|journal=Tetrahedron Asymmetry|volume=1|issue=5|language=en|pages=315–318|doi=10.1016/S0957-4166(00)86322-3|pmid=|pmc=|issn=|doi-access=free}}</ref><ref name="Hanus et al 1999">{{cite journal | vauthors = Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, Pertwee RG, Ross RA, Mechoulam R, Fride E | display-authors = 6 | title = HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 96 | issue = 25 | pages = 14228–14233 | date = December 1999 | pmid = 10588688 | pmc = 24419 | doi = 10.1073/pnas.96.25.14228 | doi-access = free | bibcode = 1999PNAS...9614228H }}</ref><ref>{{cite web |title=Properties of HU-308 ~ Formula C27H42O3 |url=http://pqr.pitt.edu/mol/CFMRIVODIXTERW-BHIFYINESA-N |website=Pitt Quantum Repository |publisher=University of Pittsburgh Department of Chemistry}}</ref> The [[chemical synthesis|synthesis]] and characterization of onternabez took place in the laboratory of [[Raphael Mechoulam]] at the [[Hebrew University of Jerusalem]] (the HU in HU-308) in the late 1990s. The [[pinene]] dimethoxy-DMH-CBD derivative onternabez was identified as a potent peripheral CB<sub>2</sub>-selective agonist in ''in vitro'' and animal studies in 1990<ref name="Mechoulam 1990" /> and 1999.<ref name="Hanus et al 1999" /> |
||
</ref><ref>{{cite web |title=Properties of HU-308 ~ Formula C27H42O3 |url=http://pqr.pitt.edu/mol/CFMRIVODIXTERW-BHIFYINESA-N |website=Pitt Quantum Repository |publisher=University of Pittsburgh Department of Chemistry}}</ref> The [[chemical synthesis|synthesis]] and characterization took place in the laboratory of Prof. [[Raphael Mechoulam|Mechoulam]] at the [[Hebrew University of Jerusalem]], (the HU in HU-308), in the late 1990s. The [[pinene]] dimethoxy-DMH-CBD derivative HU-308 was identified decades ago as a potent peripheral CB<sub>2</sub>-selective agonist in Mechoulam et al. 1990,<ref name="Mechoulam 1990" /> and in Hanus et al. 1999.<ref name="Hanus et al 1999" /> HU-308 has shown very interesting properties such as anti-inflammatory, [[analgesic]], neuroprotective, antitumor and anti-[[Osteoporosis|osteoporitic]] (anti-bone-loss) effects, and has been used as a pharmacological tool in numerous cannabinoid studies contributing to the progress in this field (e.g., Hanus et al. 1999;<ref name="Hanus et al 1999" /> Ofek et al. 2006;<ref name="Ofek et al 2006">{{cite journal| vauthors = Ofek O, Karsak M, Leclerc N, Fogel M, Frenkel B, Wright K, Tam J, Attar-Namdar M, Kram V, Shohami E, Mechoulam R, Zimmer A, Bab I|date=2006-01-17|title=Peripheral cannabinoid receptor, CB<sub>2</sub>, regulates bone mass|journal=Proc Natl Acad Sci U S A|volume=103|issue=3|language=en|pages=696–701|doi=10.1073/pnas.0504187103|pmid=16407142|pmc=1334629|issn=|doi-access=free}}</ref> Rajesh et al. 2007a,<ref name="Rafesh 2007a">{{cite journal| vauthors = Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Huffman JW, et al |date=2007-10-01|title=CB<sub>2</sub>-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion|journal=American Journal of Physiology. Heart & Circulatory Physiology|volume=293|issue=4|language=en|pages=H2210–H2218|doi=10.1152/ajpheart.00688.2007|pmid=17660390|pmc=2229632|issn=|doi-access=free}}</ref> Morales 2017<ref name="Morales">{{cite journal| vauthors = Morales P, Reggio PH, Jagerovic N |date=2017-06-28|title=An Overview on Medicinal Chemistry of Synthetic and Natural Derivatives of Cannabidiol|journal=Frontiers in Pharmacology|volume=8|issue=|language=en|pages=422|doi=10.3389/fphar.2017.00422|pmid=28701957|pmc=5487438|issn=|doi-access=free}}</ref>), including being named a pivotal advance in the NIH database in Rafesh 2007b for its discoveries, findings and results in attenuating [[oxidative stress]], [[inflammatory response]], and [[apoptosis]] (programmed cell death).<ref name="Rajesh 2007b">{{cite journal | vauthors = Rajesh M, Pan H, Mukhopadhyay P, Bátkai S, Osei-Hyiaman D, Haskó G, Liaudet L, Gao B, Pacher P | display-authors = 6 | title = Pivotal Advance: Cannabinoid-2 receptor agonist HU-308 protects against hepatic Ischemia-reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis | journal = Journal of Leukocyte Biology | volume = 82 | issue = 6 | pages = 1382–9 | date = December 2007 | pmid = 17652447 | pmc = 2225476 | doi = 10.1189/jlb.0307180 }}</ref> HU-308 is also classified as a non-steroidal anti-inflammatory drug (NSAID).<ref name="ES2784229T3 Patent">{{cite web |last1=Lynch |first1=Mary |last2=Kelly |first2=Melanie |title=Spanish Patent ES2784229T3 HU-308, HU-433, CBD-DMH Compositions and procedures for the treatment of eye inflammation and pain |url=https://patents.google.com/patent/ES2784229T3/en?oq=ES2784229T3 |website=Google Patents |publisher=Panag Pharma |access-date=22 February 2021 |quote=A61P29/00 Non-central analgesic, antipyretic or anti-inflammatory agents, e.g antirheumatic agents; Non-steroidal anti-inflammatory drugs (NSAIDs)}}</ref> |
|||
== |
==Legal status== |
||
===Osteoporosis=== |
|||
[[Cannabinoid receptors]] were first implicated in the regulation of [[bone mass]] by Karsak et al. (2004),<ref>{{cite journal| vauthors = Karsak M, Ofek O, Fogel M, Wright K, Tam J, Gabet Y, Birenboim R, Attar-Namdar M, Müller R, Cohen-Solal M |date=October 2004|title=The cannabinoid CB<sub>2</sub> receptor: a potential target for the treatment of osteoporosis|journal=Journal of Bone and Mineral Research|volume=19|issue=S1|language=en|pages=S383|doi=10.1002/jbmr.5650191306|pmid=|pmc=|issn=|doi-access=free}}</ref> who found that CB<sub>2</sub> [[knockout mice]] had markedly accelerated age-related [[Trabecular bone score|trabecular]] bone loss and [[cortex (anatomy)|cortical]] expansion accompanied by increased activity of trabecular [[osteoblast]]s, increased numbers of [[osteoclasts]], and decreased numbers of diaphyseal osteoblast precursors (Ofek et al. 2006).<ref name="Ofek et al 2006" /> CB<sub>2</sub> receptors were expressed in osteoblasts, [[osteocytes]], and osteoclasts. The selective CB<sub>2</sub> agonist HU-308, but not the [[CB1 receptor|CB]]<sub>1</sub> receptor agonist [[noladin ether]], attenuated [[ovariectomy]]-induced bone loss and markedly stimulated cortical thickness through the suppression of osteoclast number and stimulation of endocortical bone formation.<ref name="Ofek et al 2006" /> Furthermore, HU-308 dose dependently increased the number and activity of endocortical osteoblasts and restrained trabecular osteoclastogenesis by inhibiting proliferation of osteoclast precursors.<ref name="Ofek et al 2006" /> These results, coupled with CB<sub>2</sub> but not [[CB1 receptor|CB]]<sub>1</sub> receptor [[mRNA]] expression during osteoblastic differentiation, suggested a role for CB<sub>2</sub> receptors in [[bone remodeling]]. Such a role of CB<sub>2</sub> but not [[CB1 receptor|CB]]<sub>1</sub> receptors is also supported by a systematic [[Genetics|genetic]] association study by Karsak et al. (2005) in human samples of [[postmenopausal osteoporosis]] patients and matched female control subjects, which found a very statistically significant association of single [[Gene polymorphism|polymorphism]]s (P=0.0014) and [[haplotype]]s (P=0.0001) that encompassed the CNR<sub>2</sub> gene on human chromosome 1p36, while finding no convincing association for the [[psychotropic]] CNR<sub>1</sub> gene.<ref name="Karsak et al 2005">{{cite journal| vauthors = Karsak M, Cohen-Solal M, Freudenberg J, Ostertag A, Morieux C, Kornak U, Essig J, Erxlebe E, Bab I, Kubisch C, de Vernejoul MC, Zimmer A |date=15 November 2005|title=Cannabinoid receptor type 2 gene is associated with human osteoporosis|journal=Human [[Molecular Genetics]]|volume=14|issue=22|language=en|pages=3389–96|doi=10.1093/hmg/ddi370|pmid=16204352|pmc=|issn=|doi-access=free}}</ref> It is the non-psychotropic cannabinoid receptor type 2 gene that is found to be so strongly associated with human osteoporosis as P value of 0.0001.<ref name="Karsak et al 2005" /> |
|||
Onternabez is non-psychoactive and not scheduled at the federal level in the United States.<ref>{{Cite web |url=http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm |title=21 CFR — Schedules of controlled substances §1308.11 Schedule I. |access-date=2014-12-17 |archive-date=2009-08-27 |archive-url=https://web.archive.org/web/20090827043725/http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm |url-status=dead }}</ref> It is a Schedule I [[controlled substance]] in the state of [[Florida]] making it illegal to buy, sell, or possess there.<ref>{{cite web | url = http://leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0800-0899/0893/0893.html | work = Florida Statutes | title = Chapter 893 - Drug abuse prevention and control }}</ref> |
|||
===NeuroInflammation=== |
|||
HU308 promotes neural [[Progenitor cell|progenitor]] (NP) [[cell proliferation|proliferation]] and [[neurogenesis]] of [[neural stem cells]],<ref>{{cite journal | vauthors = Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzmán M, Galve-Roperh I | title = Non-psychoactive CB<sub>2</sub> cannabinoid agonists stimulate neural progenitor proliferation | journal = FASEB Journal | volume = 20 | issue = 13 | pages = 2405–7 | date = November 2006 | pmid = 17015409 | doi = 10.1096/fj.06-6164fje | s2cid = 4885167 | url = https://semanticscholar.org/paper/96b76549d320c082222bc4869d612834b1bfcfd9 }} |
|||
</ref> promotes [[neuroprotection]] and neurorepair, activates [[Phosphatidylinositol|Phosphatidyl Inositol]], and has important implications for neuronal survival under [[Neuroinflammation|neuroinflammatory]] conditions occurring in [[animal model]]s of [[neurodegenerative disease]]s, such as [[multiple sclerosis]], [[Alzheimer disease]], and [[Huntington's disease|Huntington's Disease]],<ref>{{cite journal| vauthors = Fernández-Ruiz J, González S, Romero J, Ramos JA |date=2005|title=Cannabinoids in Neurodegeneration and Neuroprotection. |journal=In: Mechoulam, R.(Ed.), Cannabinoids as Therapeutics (MDT) Birkhaüser Verlag; Switzerland|volume=|issue=|language=en|pages=79–109|issn=|doi-access=}}</ref><ref>{{cite journal| vauthors = Fernández-Ruiz J, Romero J, Velasco G, Tolón RM, Ramos JA, Guzmán M |date=Jan 2007|title=Cannabinoid CB<sub>2</sub> receptor: a new target for the control of neural cell survival|journal=Trends Pharmacol Sci|volume=28|issue=1|language=en|pages=:39–45|doi=10.1016/j.tips.2006.11.001|pmid=17141334|pmc=|issn=|doi-access=free}}</ref><ref>{{cite journal| vauthors = Esposito G, Scuderi C, Savani C, Steardo L, Jr, De Filippis D, Cottone P, Iuvone T, Cuomo V, Steardo L |date=Aug 2007|title=Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression|journal=British Journal of Pharmacology|volume=151|issue=8|language=en|pages=1272–1279|doi=10.1038/sj.bjp.0707337|pmid=17592514|pmc=2189818|issn=|doi-access=free}}</ref><ref>{{cite journal| vauthors = Fernández-López D, Pazos MR, Tolón RM, Moro MA, Romero J, Lizasoain I, Martínez-Orgado J |date=Sep 2007|title=The cannabinoid agonist WIN55212 reduces brain damage in an in vivo model of hypoxic-ischemic encephalopathy in newborn rats|journal=Pediatric Research|volume=62|issue=3|language=en|pages=255–60|doi=10.1203/PDR.0b013e318123fbb8|pmid=17622949|pmc=|issn=|doi-access=free}}</ref> and upon acute ischemic [[brain injury]].<ref>{{cite journal | vauthors = Palazuelos J, Ortega Z, Díaz-Alonso J, Guzmán M, and Galve-Roperh I | title = CB<sub>2</sub> Cannabinoid Receptors Promote Neural Progenitor Cell Proliferation via mTORC1 Signaling | journal = Journal of Biological Chemistry | volume = 287 | issue = 2 | pages = 1198–1209 | date = January 2012 | pmid = 22102284 | doi = 10.1074/jbc.M111.291294 | doi-access = free }}</ref> [[Attenuation]] of the inflammatory response in the brain has also been reported by activation of CB<sub>2</sub> receptors in a study of [[Pia mater|pial]] vessels forming the blood–brain barrier, using a model of [[lipopolysaccharide]]-induced encephalitis (Ramirez et al. 2012), wherein activation of CB<sub>2</sub> receptors decreased [[adhesion molecules]] in the brain tissue and [[Leukocyte adhesion cascade|leukocyte-endothelial adhesion]] in the pial vessels.<ref>{{cite journal| vauthors = Ramirez SH, Haskó J, Skuba A, Fan S, Dykstra H, McCormick R, Reichenbach N, Krizbai I, Mahadevan A, Zhang M, Tuma R, Son YJ, Persidsky Y |date=21 March 2012|title=Activation of cannabinoid receptor 2 attenuates leukocyte-endothelial cell interactions and blood-brain barrier dysfunction under inflammatory conditions|journal=Journal of Neuroscience|volume=32|issue=12|language=en|pages=4000–16|doi=10.1523/JNEUROSCI.4628-11.2012|pmid=22442067|pmc=3325902|issn=|doi-access=free}}</ref> HU-308 protects both [[liver]] and [[blood vessel]] tissues against hepatic ischemia and reperfusion ([[blood circulatory system]]) injury by attenuating [[oxidative stress]], [[inflammatory response]] and [[apoptosis]] via inhibition of [[TNF-α]].<ref name="Rafesh 2007a" /> The role of CB<sub>2</sub> receptors in endothelial cell activation and endothelial/inflammatory cell interactions, being critical steps not only in [[reperfusion injury]], but also [[atherosclerosis]] and other inflammatory disorders, turned out to be very important, because selective CB<sub>2</sub> cannabinoid agonist HU-308 decreased TNF-α-induced [[ICAM-1]] and [[VCAM-1]] expression in human liver [[sinusoidal endothelial cells]] (HLSECs) expressing CB<sub>2</sub> receptors, as well as the adhesion of human [[neutrophils]] to HLSECs in vitro.<ref>{{cite journal| vauthors = Pacher P, Gao B |date=Apr 2008|title=Endocannabinoids and Liver Disease. III. Endocannabinoid effects on immune cells: implications for inflammatory liver diseases|journal=Am J Physiol Gastrointest Liver Physiol|volume=294|issue=4|language=en|pages=G850–G854|doi=10.1152/ajpgi.00523.2007|pmid=18239059|pmc=2376822|issn=|doi-access=free}}</ref> HU-308 reduces blood pressure, blocks defecation, and elicits anti-inflammatory and peripheral analgesic activity.<ref name="Hanus et al 1999" /><ref>{{cite journal | vauthors = LaBuda CJ, Koblish M, Little PJ | title = Cannabinoid CB<sub>2</sub> receptor agonist activity in the hindpaw incision model of postoperative pain | journal = European Journal of Pharmacology | volume = 527 | issue = 1–3 | pages = 172–4 | date = December 2005 | pmid = 16316653 | doi = 10.1016/j.ejphar.2005.10.020 }}</ref> Currently, CBD (especially potent CBD derivatives like HU-308) generate considerable interest due to their beneficial neuroprotective, antiepileptic, anxiolytic, antipsychotic, anti-inflammatory and pain-relieving properties, therefore, the CBD scaffold becomes of increasing interest for medicinal chemists.<ref name="Morales" /> |
|||
== See also == |
|||
===Inflammation & Immune Modulation=== |
|||
* [[Cannabidiol dimethyl ether]] |
|||
HU-308 has an important functional outcome ~ the secretion of [[interleukin 6]] (IL-6) and [[interleukin 10]] (IL-10) with therapeutic [[immunomodulatory]] properties in ''vitro.''<ref>{{cite journal| vauthors = Saroz Y, Kho DT, Glass M, Graham ES, Grimsey NL |date=2019-10-19|title=Cannabinoid Receptor 2 (CB 2 ) Signals via G-alpha-s and Induces IL-6 and IL-10 Cytokine Secretion in Human Primary Leukocytes|journal=ACS Pharmacology & Translational Science|volume=2|issue=6|language=en|pages=414–428|doi=10.1021/acsptsci.9b00049|pmid=32259074|pmc=7088898|issn=2575-9108|doi-access=free}}</ref> There is evidence that IL-6 may be used as an inflammatory marker for the more severe COVID-19 infections that have a poor prognosis for a favorable outcome because raised levels of IL-6 as well as troponin are associated with a poor prognosis in COVID-19.<ref>{{cite web |title=Raised troponin and interleukin-6 levels are associated with a poor prognosis in COVID-19. 2 April 2020. Graham Cole (Imperial College Healthcare NHS Trust, London, UK) |url=https://cardiacrhythmnews.com/raised-troponin-and-interleukin-6-levels-are-associated-with-a-poor-prognosis-in-covid-19/ |website=Cardiac Rhythm News |publisher=Graham Cole, CRN |access-date=21 February 2021}}</ref> Researchers Dr. Melanie Kelly and Dr. C. Lehmann at Panag Pharma, now merged with Tetra Bio-Pharma,<ref>[https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2018/Tetra-Bio-Pharma-Enters-into-Non-Binding-Proposal-to-Acquire-Panag-Pharma-Inc/default.aspx Nov 2018, Tetra Bio-Pharma Enters into Non-Binding Proposal to Acquire Panag Pharma Inc.]</ref><ref>[https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2019/Tetra-Bio-Pharma-Enters-into-Definitive-Agreement-to-Acquire-Panag-Pharma-Inc/default.aspx Jan 2019, Tetra Bio-Pharma Enters into Definitive Agreement to Acquire Panag Pharma Inc.]</ref><ref>[https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2019/Tetra-Bio-Pharma-Shareholders-Approve-the-Acquisition-of-Panag-Pharma/default.aspx Apr 2019, Tetra Bio-Pharma Shareholders Approve the Acquisition of Panag Pharma]</ref><ref>[https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2019/Tetra-Bio-Pharma-Closesthe-Acquisition-of-Panag-Pharma/default.aspx May 2019, Tetra Bio-Pharma Closes the Acquisition of Panag Pharma]</ref> which owns the IP rights to HU-308,<ref>[http://patft.uspto.gov/netacgi/nph-Parser?Sect1=PTO2&Sect2=HITOFF&p=1&u=%2Fnetahtml%2FPTO%2Fsearch-bool.html&r=1&f=G&l=50&co1=AND&d=PTXT&s1=14722991&OS=14722991&RS=14722991 USPTO], Compositions and methods for treatment of ocular inflammation and/or pain (Lynch & Kelly Jan 2017). In certain embodiments, the non-psychotropic phytocannabinoid is beta-caryophyllene or cannabidiol [CBD] and the synthetic cannabinoid is HU-433, HU-308, or a modified CBD such as CBD-DMH.</ref><ref>[https://patents.justia.com/patent/9549906#citations Justia], Compositions and methods for treatment of ocular inflammation and/or pain (Lynch & Kelly May 2015)</ref><ref>{{cite web |last1=Lynch |first1=Mary |last2=Kelly |first2=Melanie |title=Patent 9549906 Composition & Methods for Treatment of Ocular Inflammation &/or Pain Jan 2017 |url=https://assignment.uspto.gov/patent/index.html#/patent/search/resultAbstract?id=9549906&type=patNum |website=U.S. Patent & Trademark Office |publisher=USPTO, Panag Pharma |access-date=20 February 2021}}</ref> showed with Drs. J Sardinha and J Zhou that HU 308 also mediates [[immune modulation]] in sepsis,<ref>{{cite journal | vauthors = Sardinha, Kelly, Zhou, Lehmann | title = Experimental cannabinoid 2 receptor-mediated immune modulation in sepsis | journal = Mediators of Inflammation | date = 2014 | pmid = 24803745 }}</ref> as well as displays [[antiallodynic]] activity (alleviates [[Allodynia|allodynic]] pain) in the rat hindpaw incision model of post-operative pain, is [[neuroprotective]] and improves [[Motor skill|motor performance]] in a [[Model organism|mouse model]] of [[Huntington's Disease]].<ref>https://www.tocris.com/products/hu-308_3088</ref> Continued work by Dr. MEM Kelly et al. showed HU-308 also dramatically fights the [[Cytokine release syndrome|Cytokine Release Syndrome]] (CRS), also called cytokine release storm, that is seen in many diseases and conditions, including [[Acute Respiratory Distress Syndrome]] (ARDS), [[COVID-19]], [[Sepsis]], [[Septic Shock]], [[Systemic inflammatory response syndrome|Systemic Inflammatory Response Syndrome]] (SIRS), [[Cytokine storm|Cytokine Storm Syndrome]] (CSS), [[Multiple organ dysfunction syndrome|Multi-Organ Dysfunction Syndrome]] (MODS), [[Pneumonia]], [[Uveitis]], Corneal [[Neuropathic pain|Neuropathic Pain]] [[Hyperalgesia]], [[Allodynia|Photo-allodynia]], Burning, Stinging, Dryness and [[Inflammation]]. The [[antinociceptive]] and [[anti-inflammatory]] effects of HU-308, but not Δ<sup>8</sup>THC or CBD, were mediated through CB<sub>2</sub>R, and it reduces [[cytokine storm]]s in the eye, importantly, where [[corneal]] damage can result in an [[Inflammation|inflammatory response]] that involves the production of proinflammatory cytokines, [[neovascularization]], recruitment of [[leukocytes]], and release of [[neuropeptides]] producing inflammatory pain.<ref>https://www.cas.org/blog/covid-19-cytokine-storms</ref><ref>https://pubmed.ncbi.nlm.nih.gov/31613449/</ref><ref name="Thapa">{{cite journal | vauthors = Thapa, Cairns, Szczesniak, Toguri, Caldwell, Kelly | title = The Cannabinoids Δ8THC, CBD, and HU-308 Act via Distinct Receptors to Reduce Corneal Pain and Inflammation | journal = Cannabis & Cannabinoid Research | date = 2018 | pmid = 29450258}}</ref> The Thapa et al. study on HU-308 in reducing Corneal Pain in 2018 is the first time a CB<sub>2</sub>R agonist has been demonstrated to reduce corneal pain.<ref name="Thapa" /> HU-308 is a selective and highly potent agonist at CB<sub>2</sub>R and has previously been shown to reduce [[lipopolysaccharide]]-induced intraocular inflammation.<ref name="Thapa" /><ref>{{cite journal | vauthors = Toguri, Lehmann, Laprairie, Szczesniak, Zhou, Denovan-Wright, Kelly | display-authors = | title = Anti-inflammatory effects of cannabinoid CB(2) receptor activation in endotoxin-induced uveitis | journal = British Journal of Pharmacology | volume = 171 | issue = 6 | pages = 1448–61 | date = March 2014 | pmid = 24308861 | pmc = 3954484 | doi = 10.1111/bph.12545 }}</ref> |
|||
* [[Cannabidiol diacetate]] |
|||
* [[HU-210]] |
|||
===Multi-Organ Dysfunction & Damage=== |
|||
* [[HU-320]] |
|||
While CB<sub>2</sub> [[knockout mice]] developed enhanced inflammation and tissue injury from [[cisplatin]]-induced [[kidney damage]], HU-308, working through the [[endocannabinoid system]] and the CB<sub>2</sub> receptor, protected against cisplatin-induced kidney damage by attenuating inflammation and [[Oxidative stress|oxidative]] or [[nitrosative stress]], and such selective CB<sub>2</sub> agonists may represent a promising novel approach to prevent this devastating complication of [[chemotherapy]].<ref name="Mukhopadhyay">{{cite journal | vauthors = Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Bátkai S, Gao B, Haskó G, Pacher P | display-authors = 7 | title = Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress and cell death in nephropathy | journal = Free Radical Biology and Medicine | volume = 48 | issue = 3 | pages = 457–67 | date = February 2010 | pmid = 19969072 | pmc = 2869084 | doi = 10.1016/j.freeradbiomed.2009.11.022 }}</ref> Activation of the cannabinoid-2 (CB<sub>2</sub>) receptors (expressed predominantly in [[immune cells]], and also to a much less extent in other cell types, e.g., endothelial and [[parenchymal]] cells) by recently recognized endogenous [[lipid]] mediators (the endocannabinoids) produced and present in virtually all tissues/organ systems,<ref>{{cite journal | vauthors = Mechoulam R, Fride E, DiMarzo V | display-authors = | title = Endocannabinoids. | journal = Eur J Pharmacol. | volume = 359 | issue = 1 | pages = 1–18 | date = 1998 | pmid = 9831287 | pmc = | doi = 10.1016/s0014-2999(98)00649-9 }}</ref><ref>{{cite journal | vauthors = Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG | display-authors = | title = International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. | journal = Pharmacol Rev | volume = 54 | issue = 2 | pages = 161–202 | date = 2002 | pmid = 12037135 | pmc = | doi = 10.1124/pr.54.2.161 }}</ref><ref>{{cite journal | vauthors = Pacher P, Batkai S, Kunos G | display-authors = | title = International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. The Endocannabinoid System as an Emerging Target of Pharmacotherapy | journal = Pharmacol Rev | volume = 58 | issue = 3 | pages = 389–462 | date = Sep 2006 | pmid = 16968947 | pmc = 2241751 | doi = 10.1124/pr.58.3.2 }}</ref> or by selective synthetic CB<sub>2</sub> agonists such as HU-308 in the pivotal advance by Rajesh et al. (2007),<ref name="Rajesh 2007b" /> has been shown to protect against tissue damage in various experimental models of ischemic-reperfusion injury,<ref name="Rajesh 2007b" /><ref>{{cite journal| vauthors = Batkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, Hong F, Harvey-White J, Jafri A, Hasko G, Huffman JW, Gao B, Kunos G, Pacher P |date=Jun 2007|title=Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury|journal=FASEB J|volume=21|issue=8|language=en|pages=1788–1800|doi=10.1096/fj.06-7451com|pmid=17327359|pmc=2228252|issn=|doi-access=free}}</ref> atherosclerosis/cardiovascular inflammation,<ref>{{cite journal| vauthors = Gallily R, Breuer A, Mechoulam R |date=2000-10-06|title=2-Arachidonylglycerol, an endogenous cannabinoid, inhibits tumor necrosis factor-alpha production in murine macrophages, and in mice|journal=Eur J Pharmacol|volume=406|issue=1|language=en|pages=R5-7|doi=10.1016/s0014-2999(00)00653-1|pmid=11011050|pmc=|doi-access=free}}</ref><ref>{{cite journal| vauthors = Gaoni Y, Mechoulam R |date=1971-01-13|title=The isolation and structure of delta-1-tetrahydrocannabinol and other neutral cannabinoids from hashish|journal=J Am Chem Soc|volume=93|issue=1|language=en|pages=217–24|doi=10.1021/ja00730a036|pmid=5538858|pmc=|issn=|doi-access=free}}</ref><ref>{{cite journal| vauthors = García-Arencibia M, González S, de Lago E, Ramos JA, Mechoulam R, Fernández-Ruiz J |date=2007-02-23|title=Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: importance of antioxidant and cannabinoid receptor-independent properties|journal=Brain Res|volume=1134|issue=1|language=en|pages=162–70|doi=10.1016/j.brainres.2006.11.063|pmid=17196181|pmc=|issn=|doi-access=free}}</ref> and neurodegenerative,<ref>{{cite journal| vauthors = Sagredo O, González S, Aroyo I, Pazos M, Benito C, Lastres-Becker I, Romero J, Tolón R, Mechoulam R, Brouillet E, Romero J, Fernández-Ruiz J |date=2009-08-15|title=Cannabinoid CB<sub>2</sub> receptor agonists protect the striatum against malonate toxicity: Relevance for Huntington’s disease|journal=Glia|volume=57|issue=11|language=en|pages=1154–67|doi=10.1002/glia.20838|pmid= |pmc=2706932 }}</ref> [[gastrointestinal]]<ref name="Ke 2016">{{cite journal| vauthors = Ke P, Shao BZ, Xu ZQ, et al |date=2016-09-09|title=Activation of Cannabinoid Receptor 2 Ameliorates DSS-Induced Colitis through Inhibiting NLRP3 Inflammasome in Macrophages|journal=PLoS One|volume=11|issue=9|language=en|pages=e0155076|doi=10.1371/journal.pone.0155076|pmid=27611972|pmc=5017608|issn=|doi-access=free}}</ref><ref>{{cite journal| vauthors = Storr MA, Keenan CM, Zhang H, Patel KD, Makriyannis A, Sharkey KA |date=Nov 2009|title=Activation of the cannabinoid 2 receptor (CB(2)) protects against experimental colitis|journal=Inflammable Bowel Disease|volume=15|issue=11|language=en|pages=1678–1685|doi=10.1002/ibd.20960|pmid=19408320|pmc=5531765|issn=|doi-access=free}}</ref> and other disorders by limiting inflammatory cell [[chemotaxis]]/[[Infiltration (medical)|infiltration]], activation and interrelated oxidative/nitrosative stress.<ref>{{cite journal| vauthors = Pacher P, Batkai S, Kunos G |date=Sep 2006|title=The endocannabinoid system as an emerging target of pharmacotherapy|journal=Pharmacol Rev.|volume=58|issue=3|language=en|pages=389–462|doi=10.1124/pr.58.3.2|pmid=16968947|pmc=2241751|issn=|doi-access=free}}</ref><ref>{{cite journal| vauthors = Bátkai S, Járai Z, Wagner JA, Goparaju SK, Varga K, Liu J, Wang L, Mirshahi F, Khanolkar AD, Makriyannis A, Urbaschek R, Garcia N Jr, Sanyal AJ, Kunos G |date=July 2001|title=Endocannabinoids acting at vascular CB1 receptors mediate the vasodilated state in advanced liver cirrhosis|journal=Nat Med|volume=7|issue=7|language=en|pages=827–32|doi=10.1038/89953|pmid=11433348|pmc=|issn=|doi-access=free}}</ref><ref>{{cite journal| vauthors = Engeli S, Böhnke J, Feldpausch M, Gorzelniak K, Janke J, Bátkai S, Pacher P, Harvey-White J, Luft FC, Sharma AM, Jordan J |date=October 2005|title=Activation of the Peripheral Endocannabinoid System in Human Obesity|journal=Diabetes|volume=54|issue=10|language=en|pages=2838–2843|doi=10.2337/diabetes.54.10.2838|pmid=16186383|pmc=2228268|issn=|doi-access=free}}</ref><ref>{{cite journal| vauthors = Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L |date=May 2005|title=Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity|journal=J Clin Invest|volume=115|issue=5|language=en|pages=1298–305|doi=10.1172/JCI23057|pmid=15864349|pmc=1087161|issn=|doi-access=free}}</ref> In vivo, HU308 treatment attenuated DSS-induced [[colitis]] mice associated with reduced colon inflammation and inhibited [[NLRP3]] [[inflammasome]] activation in wild-type mice.<ref name="Ke 2016" /> Furthermore, CB<sub>2</sub> receptors are over-expressed in a variety of cancers, and CB<sub>2</sub> activation may decrease the proliferation and growth of various [[cancer cells]] and [[tumors]].<ref name="Mukhopadhyay" /><ref>{{cite journal| vauthors = Kunikowska G, Jenner P |date=2001-12-13|title=6-Hydroxydopamine-lesioning of the nigrostriatal pathway in rats alters basal ganglia mRNA for copper, zinc- and manganese-superoxide dismutase, but not glutathione peroxidase|journal=Brain Res|volume=922|issue=1|language=en|pages=51–64|doi=10.1016/s0006-8993(01)03149-3|pmid=11730701|pmc=|issn=|doi-access=free}}</ref> HU-308 was shown to reduce swelling, [[Synovial joint|synovial join inflammation and destruction]], in addition to lowering circulating [[antibodies]] against [[Collagen I]].<ref>{{cite journal| vauthors = Gui H, Liu X, Liu LR, Su DF, Dai SM |date=Jun 2015|title=Activation of cannabinoid receptor 2 attenuates synovitis and joint distruction in collagen-induced arthritis|journal=Immunobiology|volume=220|issue=6|language=en|pages=817–22|doi=10.1016/j.imbio.2014.12.012|pmid=25601571|pmc=|issn=|doi-access=free}}</ref> |
|||
* [[HU-211]] |
|||
* [[Nabilone]] |
|||
===ARDS-003=== |
|||
* [[CP 47,497]] |
|||
HU-308, aka ARDS-03 for its ARDS fighting abilities, is currently in a collaboration study by Tetra Bio-Pharma, Targeted Pharmaceutical, LLC, [[George Mason University]] and the [[NIH]] at the university's top-level National Center for Biodefense and Infectious Diseases Biomedical Research Laboratory (BRL) against the lethal condition [[Acute Respiratory Distress Syndrome]] (ARDS) seen in [[COVID-19]] patients.<ref name="forbes1">https://www.forbes.com/sites/emilyearlenbaugh/2020/08/20/synthetic-cannabinoid-drug-for-covid-19-approved-for-phase-1-clinical-trials/</ref><ref>https://s24.q4cdn.com/136309390/files/doc_presentation/2020/12/Tetra-Bio-Pharma-Milestones-Update-Dec.-30-2020.pdf</ref><ref>https://www.sedar.com/GetFile.do?lang=EN&docClass=7&issuerNo=00026458&issuerType=03&projectNo=03122925&docId=4813488</ref><ref>https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2020/Tetra-Bio-Pharma-Targeted-Pharmaceutical--the-George-Mason-University-Partner-on-ARDS-003-to-Prevent--Treat-COVID-19/default.aspx</ref><ref name="Liotta">https://science.gmu.edu/directory/lance-liotta</ref><ref>https://tetrabiopharma.com/partners/</ref> Regulatory filings show that in late 2020 Tetra and Targeted designed short- to mid- term studies to gather additional data on the benefits of ARDS-003 in Sars-CoV-2 infected animal models for the prevention of ARDS in COVID-19.<ref name="SEDAR-17-2-2021">{{cite web |title=SEDAR TBP Annual Report 17 Feb 2021 |url=https://www.sedar.com/GetFile.do?lang=EN&docClass=7&issuerNo=00026458&issuerType=03&projectNo=03174069&docId=4888388&fbclid=IwAR3W82_NAVXYU0QVVyDBfWrZi7qW7KMBol6cweldmB3GXNTlggMrANeKdbo |website=SEDAR |publisher=SEDAR Tetra Bio-Pharma |access-date=20 February 2021}}</ref> A former Deputy Director of the NIH is heading the GMU research on ARDS-003, which is a novel, sterile, injectable, optimized, [[nanoemulsion]] form of HU-308 that has successfully undergone stringent safety and [[toxicology]] studies in accordance with U.S. FDA oversight, which were required before submitting an [[investigational new drug]] (IND) application in the US and a clinical trial application (CTA) in Canada for a [[Phase 1 clinical testing|Phase 1 research study]] through the Sars-CoV-2 regulatory fast track pathway.<ref name="Liotta" /><ref name="TBP ARDS3 News">https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2020/Tetra-Bio-Pharma-Completes-Major-Milestone-for-COVID-19-Therapeutic/default.aspx</ref> The toxicology program was designed to the standards of the [[International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use|International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use]] (ICH) for enabling a [[First-in-human trial|first-in-human]] clinical trial; and included general toxicology data for two species-specific studies to assess toxicity in major organ systems ([[cardiovascular]], [[respiratory]], [[nervous system]]) and [[genotoxicity]], as well as the [[metabolism]] and [[Pharmacokinetics|pharmacokinetic]] distribution of the drug.<ref name="TBP ARDS3 News" /> Tetra Bio-Pharma is the first [[endocannabinoid system]] (ECS) biotechnology company researching a cannabinoid treatment for ARDS and sepsis linked to COVID-19, pneumonia and other critical conditions, and the ARDS-003 pharmaceutical drug now has FDA approval to begin Phase I and Phase II clinical trials in human subjects for the reduction of cytokine storm, sepsis, and ARDS in COVID-19.<ref name="forbes1" /><ref name="TBP ARDS3 News" /> [[George Mason University Notable Faculty and Alumni|GMU]] researchers including the Co-Director, Center for Applied [[Proteomics]] and [[Molecular medicine|Molecular Medicine]] (CAPMM), are conducting three studies to assess the therapeutic efficacy of candidate interventions for COVID-19 in mouse models of [[Angiotensin-converting enzyme 2 |angiotensin-converting enzyme 2 (ACE2)]] animals infected [[Nasal administration|intranasally]] with [[SARS-CoV-2]] to determine the survival advantage conferred by a therapeutic, to determine the survival advantage conferred by a therapeutic if an alternate course or dosing strategy needs to be followed, and to determine viral levels on day three post-infection when [[viral load]] in the [[lung]]s is expected to peak.<ref>{{cite web |last1=Narayanan |first1=Aarthi |last2=Liotta |first2=Lance |title=GMU Grant Announcement: Narayanan and Liotta testing therapeutic efficacy of potential COVID-19 treatments |url=https://www.eurekalert.org/pub_releases/2021-02/gmu-nl021921.php |website=EurekAlert! operated by the nonprofit American Association for the Advancement of Science (AAAS) |publisher=George Mason University |access-date=24 February 2021}}</ref> Dalton Pharma is producing the injectable drug for the GMU effort.<ref>{{cite web |last1=Cachapero |first1=Joanne |title=Cannabis and Coronavirus: Sales Surge as the Industry Carries On |url=https://mgretailer.com/cannabis-news/cannabis-and-coronavirus-sales-surge-as-the-industry-carries-on/ |website=MG Retailer |access-date=28 February 2021 |quote=Canadian cannabis pharmaceutical company Tetra Bio-Pharma recently contracted with Dalton Pharma Services to produce batches of its HU-308 and ARDS-003, which could help to treat severe cytokine reactions.}}</ref> |
|||
==Legal status== |
|||
HU-308 is non-psychoactive and not scheduled at the federal level in the United States.<ref>[http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm 21 CFR — Schedules of controlled substances §1308.11 Schedule I.]</ref> It is a Schedule I [[controlled substance]] in the state of [[Florida]] making it illegal to buy, sell, or possess there.<ref>[http://leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0800-0899/0893/0893.html Florida Statutes - Chapter 893 - Drug abuse prevention and control]</ref> |
|||
== References == |
== References == |
||
{{reflist}} |
{{reflist}} |
||
{{Cannabinoids}} |
{{Cannabinoids}} |
||
== See also == |
|||
* [[HU-210]] |
|||
* [[HU-320]] |
|||
[[Category:Primary alcohols]] |
[[Category:Primary alcohols]] |
||
Latest revision as of 08:24, 16 October 2024
 | |
| Clinical data | |
|---|---|
| Other names | HU-308, HU308, PPP-003, ARDS-003 |
| Drug class | CB2 receptor agonist |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Excretion | Kidneys |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H42O3 |
| Molar mass | 414.630 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Onternabez (also known as HU-308, HU308, PPP-003, and ARDS-003) is a synthetic cannabinoid that acts as a potent cannabinoid agonist. It is highly selective for the cannabinoid-2 receptor (CB2 receptor) subtype, with a selectivity more than 5,000 times greater for the CB2 receptor than the CB1 receptor.[1][2][3] The synthesis and characterization of onternabez took place in the laboratory of Raphael Mechoulam at the Hebrew University of Jerusalem (the HU in HU-308) in the late 1990s. The pinene dimethoxy-DMH-CBD derivative onternabez was identified as a potent peripheral CB2-selective agonist in in vitro and animal studies in 1990[1] and 1999.[2]
Legal status
[edit]Onternabez is non-psychoactive and not scheduled at the federal level in the United States.[4] It is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess there.[5]
See also
[edit]References
[edit]- ^ a b Mechoulam R, Lander N, Breuer A, Zahalka J (1990-04-11). "Synthesis of the individual, pharmacologically distinct enantiomers of a tetrahydrocannabinol derivative". Tetrahedron Asymmetry. 1 (5): 315–318. doi:10.1016/S0957-4166(00)86322-3.
- ^ a b Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. (December 1999). "HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor". Proceedings of the National Academy of Sciences of the United States of America. 96 (25): 14228–14233. Bibcode:1999PNAS...9614228H. doi:10.1073/pnas.96.25.14228. PMC 24419. PMID 10588688.
- ^ "Properties of HU-308 ~ Formula C27H42O3". Pitt Quantum Repository. University of Pittsburgh Department of Chemistry.
- ^ "21 CFR — Schedules of controlled substances §1308.11 Schedule I." Archived from the original on 2009-08-27. Retrieved 2014-12-17.
- ^ "Chapter 893 - Drug abuse prevention and control". Florida Statutes.
