Onternabez: Difference between revisions
David notMD (talk | contribs) →Neuroinflammation: all refs either in vitro, animal model, or review about cannabidiol in general, so entire section deleted |
Slothwizard (talk | contribs) No edit summary Tags: Visual edit Mobile edit Mobile web edit |
||
| (21 intermediate revisions by 11 users not shown) | |||
| Line 1: | Line 1: | ||
{{Undisclosed paid|date=November 2021}} |
|||
{{short description|Cannabidiol-derivative drug}} |
{{short description|Cannabidiol-derivative drug}} |
||
{{Drugbox |
{{Drugbox |
||
| Verifiedfields |
| Verifiedfields = changed |
||
| Watchedfields |
| Watchedfields = changed |
||
| class = [[CB2 receptor|CB<sub>2</sub> receptor agonist]] |
|||
| verifiedrevid = 426291380 |
|||
| verifiedrevid = 426291380 |
|||
| IUPAC_name = [(1''R'',2''R'',5''R'')-2-[2,6-Dimethoxy-4-(2-methyloctan-2-yl)phenyl]-7,7-dimethyl-4-bicyclo[3.1.1]hept-3-enyl]methanol |
|||
| IUPAC_name = [(1''S'',2''S'',5''S'')-2-[2,6-Dimethoxy-4-(2-methyloctan-2-yl)phenyl]-7,7-dimethyl-4-bicyclo[3.1.1]hept-3-enyl]methanol |
|||
| image = HU-308.png |
|||
| image = Onternabez.svg |
|||
| caption = |
|||
| caption = |
|||
| width = |
|||
| width = <!--Clinical data--> |
|||
| tradename = |
|||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|||
| pregnancy_category = |
|||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> |
|||
| legal_CA = Schedule II |
|||
| legal_UK = Class B |
|||
| legal_US = Unscheduled <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> <BR> |
|||
| legal_US_comment = |
|||
| legal_status = Florida: Schedule I |
|||
| routes_of_administration = <!--Pharmacokinetic data--> |
|||
| bioavailability = |
|||
| protein_bound = |
|||
| metabolism = [[Liver]] |
|||
| elimination_half-life = |
|||
| excretion = Kidneys |
|||
<!--Identifiers-->| CAS_number_Ref = {{cascite|correct|CAS}} |
|||
<!--Clinical data--> |
|||
| CAS_number = 256934-39-1 |
|||
| tradename = |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
|||
| UNII = 8I5L034D55 |
|||
| pregnancy_category = |
|||
| ATC_prefix = None |
|||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> |
|||
| ATC_suffix = |
|||
| legal_CA = Schedule II |
|||
| PubChem = 11553430 |
|||
| legal_UK = Class B |
|||
| ChEBI = 146244 |
|||
| legal_US = Unscheduled <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> <BR> |
|||
| synonyms = HU-308, HU308, PPP-003, ARDS-003 |
|||
| legal_US_comment = |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| legal_status = Florida: Schedule I |
|||
| DrugBank = |
|||
| routes_of_administration = |
|||
| KEGG = D12305 |
|||
| ChemSpiderID = 8020425 |
|||
<!--Pharmacokinetic data--> |
|||
<!--Chemical data-->| C = 27 |
|||
| bioavailability = |
|||
| H = 42 |
|||
| protein_bound = |
|||
| O = 3 |
|||
| metabolism = [[Liver]] |
|||
| smiles = CCCCCCC(C)(C)C1=CC(=C(C(=C1)OC)[C@H]2C=C([C@@H]3C[C@H]2C3(C)C)CO)OC |
|||
| elimination_half-life = |
|||
| StdInChI = 1S/C27H42O3/c1-8-9-10-11-12-26(2,3)19-14-23(29-6)25(24(15-19)30-7)20-13-18(17-28)21-16-22(20)27(21,4)5/h13-15,20-22,28H,8-12,16-17H2,1-7H3/t20-,21-,22+/m0/s1 |
|||
| excretion = Kidneys |
|||
| StdInChIKey = CFMRIVODIXTERW-FDFHNCONSA-N |
|||
<!--Identifiers--> |
|||
| CAS_number_Ref = {{cascite|changed|CAS}} |
|||
| CAS_number = 256934-39-1 |
|||
| UNII_Ref = {{fdacite|changed|FDA}} |
|||
| UNII = 8I5L034D55 |
|||
| ATC_prefix = |
|||
| ATC_suffix = |
|||
| PubChem = 5311172 |
|||
| ChEBI = 146244 |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| DrugBank = |
|||
| ChemSpiderID = 8020425 |
|||
<!--Chemical data--> |
|||
| C = 27 | H = 42 | O = 3 |
|||
| smiles = CCCCCCC(C)(C)C1=CC(=C(C(=C1)OC)[C@H]2C=C([C@@H]3C[C@H]2C3(C)C)CO)OC |
|||
| StdInChI = 1S/C27H42O3/c1-8-9-10-11-12-26(2,3)19-14-23(29-6)25(24(15-19)30-7)20-13-18(17-28)21-16-22(20)27(21,4)5/h13-15,20-22,28H,8-12,16-17H2,1-7H3/t20-,21-,22+/m0/s1 |
|||
| StdInChIKey = CFMRIVODIXTERW-FDFHNCONSA-N |
|||
}} |
}} |
||
''' |
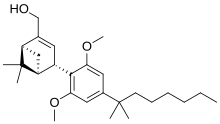
'''Onternabez''' (also known as '''HU-308''', '''HU308''', '''PPP-003''', and '''ARDS-003''') is a [[Synthetic cannabinoids|synthetic cannabinoid]] that acts as a potent [[cannabinoid]] [[agonist]]. It is highly selective for the [[cannabinoid receptor type 2|cannabinoid-2 receptor]] (CB<sub>2</sub> receptor) subtype, with a selectivity more than 5,000 times greater for the CB<sub>2</sub> receptor than the [[CB1 receptor|CB<sub>1</sub> receptor]].<ref name="Mechoulam 1990">{{cite journal| vauthors = Mechoulam R, Lander N, Breuer A, Zahalka J |date=1990-04-11|title=Synthesis of the individual, pharmacologically distinct enantiomers of a tetrahydrocannabinol derivative|journal=Tetrahedron Asymmetry|volume=1|issue=5|language=en|pages=315–318|doi=10.1016/S0957-4166(00)86322-3|pmid=|pmc=|issn=|doi-access=free}}</ref><ref name="Hanus et al 1999">{{cite journal | vauthors = Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, Pertwee RG, Ross RA, Mechoulam R, Fride E | display-authors = 6 | title = HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 96 | issue = 25 | pages = 14228–14233 | date = December 1999 | pmid = 10588688 | pmc = 24419 | doi = 10.1073/pnas.96.25.14228 | doi-access = free | bibcode = 1999PNAS...9614228H }}</ref><ref>{{cite web |title=Properties of HU-308 ~ Formula C27H42O3 |url=http://pqr.pitt.edu/mol/CFMRIVODIXTERW-BHIFYINESA-N |website=Pitt Quantum Repository |publisher=University of Pittsburgh Department of Chemistry}}</ref> The [[chemical synthesis|synthesis]] and characterization of onternabez took place in the laboratory of [[Raphael Mechoulam]] at the [[Hebrew University of Jerusalem]] (the HU in HU-308) in the late 1990s. The [[pinene]] dimethoxy-DMH-CBD derivative onternabez was identified as a potent peripheral CB<sub>2</sub>-selective agonist in ''in vitro'' and animal studies in 1990<ref name="Mechoulam 1990" /> and 1999.<ref name="Hanus et al 1999" /> |
||
</ref><ref>{{cite web |title=Properties of HU-308 ~ Formula C27H42O3 |url=http://pqr.pitt.edu/mol/CFMRIVODIXTERW-BHIFYINESA-N |website=Pitt Quantum Repository |publisher=University of Pittsburgh Department of Chemistry}}</ref> The [[chemical synthesis|synthesis]] and characterization of HU-308 took place in the laboratory of [[Raphael Mechoulam]] at the [[Hebrew University of Jerusalem]] (the HU in HU-308) in the late 1990s. The [[pinene]] dimethoxy-DMH-CBD derivative HU-308 was identified as a potent peripheral CB<sub>2</sub>-selective agonist in ''in vitro'' and animal studies in 1990<ref name="Mechoulam 1990" /> and 1999.<ref name="Hanus et al 1999" /> |
|||
==Pharmacology== |
|||
===ARDS-003=== |
|||
HU-308 is also known as ARDS-03 for its ARDS-fighting abilities. A collaboration study at the US [[National Institutes of Health]] (NIH) at [[George Mason University]]'s (GMU's) National Center for Biodefense and Infectious Diseases Biomedical Research Laboratory (BRL) is examining ways to prevent lethal ARDS seen in [[COVID-19]] patients.<ref name="forbes1">{{cite web |url=https://www.forbes.com/sites/emilyearlenbaugh/2020/08/20/synthetic-cannabinoid-drug-for-covid-19-approved-for-phase-1-clinical-trials/?sh=83ef66533298 |title=Synthetic Cannabinoid Drug For Covid-19 Approved For Phase-1 Clinical Trials |last=Earlenbaugh |first=Emily |date=August 20, 2020 |website=Forbes |publisher= |access-date=}}</ref><ref>https://s24.q4cdn.com/136309390/files/doc_presentation/2020/12/Tetra-Bio-Pharma-Milestones-Update-Dec.-30-2020.pdf</ref><ref>{{Cite web|url=https://www.sedar.com/GetFile.do?lang=EN&docClass=7&issuerNo=00026458&issuerType=03&projectNo=03122925&docId=4813488|title = Download SEDAR Filings - Code Verification and Accept Terms of Use}}</ref><ref>{{cite press release |author=<!--Not stated--> |title=Tetra Bio-Pharma, Targeted Pharmaceutical & the George Mason University Partner on ARDS-003 to Prevent & Treat COVID-19 |url=https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2020/Tetra-Bio-Pharma-Targeted-Pharmaceutical--the-George-Mason-University-Partner-on-ARDS-003-to-Prevent--Treat-COVID-19/default.aspx |location=Ottawa, Ontario |publisher=Tetra Bio-Pharma |agency= |date=October 22, 2020 |access-date=}}</ref><ref name="Liotta">{{cite web |url=https://science.gmu.edu/directory/lance-liotta |title=Lance Liotta |website=GMU College of Science}}</ref><ref>{{Cite web|url=https://tetrabiopharma.com/partners/|title=Partners – Tetra Bio-Pharma}}</ref> Regulatory filings show that in late 2020, Tetra Bio-Pharma and Targeted Pharmaceutical designed short-to-mid-term studies to gather additional data on the benefits of ARDS-003 in [[SARS-CoV-2]] infected animal models for the prevention of ARDS in COVID-19.<ref name="SEDAR-17-2-2021">{{cite web |title=SEDAR TBP Annual Report 17 Feb 2021 |url=https://www.sedar.com/GetFile.do?lang=EN&docClass=7&issuerNo=00026458&issuerType=03&projectNo=03174069&docId=4888388&fbclid=IwAR3W82_NAVXYU0QVVyDBfWrZi7qW7KMBol6cweldmB3GXNTlggMrANeKdbo |website=SEDAR |publisher=SEDAR Tetra Bio-Pharma |access-date=20 February 2021}}</ref> A former NIH deputy director is heading the GMU research on ARDS-003, which is a novel, sterile, injectable, optimized, [[nanoemulsion]] form of HU-308 that has successfully undergone safety and [[toxicology]] studies in accordance with USFDA oversight, which were required before submitting an [[investigational new drug]] (IND) application in the US and a clinical trial application (CTA) in Canada for a [[Phase 1 clinical testing|Phase 1 research study]] through the SARS-CoV-2 regulatory fast-track pathway.<ref name="Liotta" /><ref name="TBP ARDS3 News">{{Cite web|url=https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2020/Tetra-Bio-Pharma-Completes-Major-Milestone-for-COVID-19-Therapeutic/default.aspx|title = Tetra Bio-Pharma Completes Major Milestone for COVID-19 Therapeutic}}</ref> The toxicology program was designed to the standards of the [[International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use|International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use]] (ICH) for enabling a [[First-in-human trial|first-in-human]] clinical trial; and included general toxicology data for two species-specific studies to assess toxicity in major organ systems (cardiovascular, respiratory, and nervous system) and [[genotoxicity]], as well as the [[metabolism]] and [[Pharmacokinetics|pharmacokinetic]] distribution of the drug.<ref name="TBP ARDS3 News" /> Tetra Bio-Pharma is the first [[endocannabinoid system]] (ECS) biotechnology company researching a cannabinoid treatment for ARDS and sepsis linked to COVID-19, pneumonia and other critical conditions, The ARDS-003 pharmaceutical drug gained FDA approval to begin Phase I and Phase II clinical trials in human subjects for the reduction of cytokine storm, sepsis, and ARDS in COVID-19.<ref name="forbes1" /><ref name="TBP ARDS3 News" /> GMU researchers are conducting three studies to assess the therapeutic efficacy of candidate interventions for COVID-19 in mouse models of [[angiotensin-converting enzyme 2]] (ACE2) animals infected [[Nasal administration|intranasally]] with SARS-CoV-2 to determine the survival advantage conferred by a therapeutic if an alternate course or dosing strategy needs to be followed, and to determine viral levels on day-three post-infection when [[viral load]] in the lungs is expected to peak.<ref>{{cite web |last1=Narayanan |first1=Aarthi |last2=Liotta |first2=Lance |title=GMU Grant Announcement: Narayanan and Liotta testing therapeutic efficacy of potential COVID-19 treatments |url=https://www.eurekalert.org/pub_releases/2021-02/gmu-nl021921.php |website=EurekAlert! operated by the nonprofit American Association for the Advancement of Science (AAAS) |publisher=George Mason University |access-date=24 February 2021}}</ref> Dalton Pharma is producing the injectable drug for the GMU effort.<ref>{{cite web |last1=Cachapero |first1=Joanne |title=Cannabis and Coronavirus: Sales Surge as the Industry Carries On |url=https://mgretailer.com/cannabis-news/cannabis-and-coronavirus-sales-surge-as-the-industry-carries-on/ |website=MG Retailer |date=27 August 2020 |access-date=28 February 2021 |quote=Canadian cannabis pharmaceutical company Tetra Bio-Pharma recently contracted with Dalton Pharma Services to produce batches of its HU-308 and ARDS-003, which could help to treat severe cytokine reactions.}}</ref> |
|||
==Legal status== |
==Legal status== |
||
Tetra Bio-Pharma owns the intellectual property rights to HU-308.<ref>{{cite web |last1=Lynch |first1=Mary |last2=Kelly |first2=Melanie |title=Patent 9549906 Composition & Methods for Treatment of Ocular Inflammation &/or Pain Jan 2017 |url=https://assignment.uspto.gov/patent/index.html#/patent/search/resultAbstract?id=9549906&type=patNum |website=U.S. Patent & Trademark Office |publisher=USPTO, Panag Pharma |access-date=20 February 2021}}</ref><ref>[https://ir.tetrabiopharma.com/newsroom/press-releases/news-details/2019/Tetra-Bio-Pharma-Closesthe-Acquisition-of-Panag-Pharma/default.aspx May 2019, Tetra Bio-Pharma Closes the Acquisition of Panag Pharma]</ref> |
|||
Onternabez is non-psychoactive and not scheduled at the federal level in the United States.<ref>{{Cite web |url=http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm |title=21 CFR — Schedules of controlled substances §1308.11 Schedule I. |access-date=2014-12-17 |archive-date=2009-08-27 |archive-url=https://web.archive.org/web/20090827043725/http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm |url-status=dead }}</ref> It is a Schedule I [[controlled substance]] in the state of [[Florida]] making it illegal to buy, sell, or possess there.<ref>{{cite web | url = http://leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0800-0899/0893/0893.html | work = Florida Statutes | title = Chapter 893 - Drug abuse prevention and control }}</ref> |
|||
== See also == |
|||
* [[Cannabidiol dimethyl ether]] |
|||
* [[Cannabidiol diacetate]] |
|||
* [[HU-210]] |
|||
* [[HU-320]] |
|||
* [[HU-211]] |
|||
* [[Nabilone]] |
|||
* [[CP 47,497]] |
|||
== References == |
== References == |
||
{{reflist}} |
{{reflist}} |
||
{{Cannabinoids}} |
{{Cannabinoids}} |
||
== See also == |
|||
* [[HU-210]] |
|||
* [[HU-320]] |
|||
[[Category:Primary alcohols]] |
[[Category:Primary alcohols]] |
||
Latest revision as of 08:24, 16 October 2024
 | |
| Clinical data | |
|---|---|
| Other names | HU-308, HU308, PPP-003, ARDS-003 |
| Drug class | CB2 receptor agonist |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Excretion | Kidneys |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H42O3 |
| Molar mass | 414.630 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Onternabez (also known as HU-308, HU308, PPP-003, and ARDS-003) is a synthetic cannabinoid that acts as a potent cannabinoid agonist. It is highly selective for the cannabinoid-2 receptor (CB2 receptor) subtype, with a selectivity more than 5,000 times greater for the CB2 receptor than the CB1 receptor.[1][2][3] The synthesis and characterization of onternabez took place in the laboratory of Raphael Mechoulam at the Hebrew University of Jerusalem (the HU in HU-308) in the late 1990s. The pinene dimethoxy-DMH-CBD derivative onternabez was identified as a potent peripheral CB2-selective agonist in in vitro and animal studies in 1990[1] and 1999.[2]
Legal status
[edit]Onternabez is non-psychoactive and not scheduled at the federal level in the United States.[4] It is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess there.[5]
See also
[edit]References
[edit]- ^ a b Mechoulam R, Lander N, Breuer A, Zahalka J (1990-04-11). "Synthesis of the individual, pharmacologically distinct enantiomers of a tetrahydrocannabinol derivative". Tetrahedron Asymmetry. 1 (5): 315–318. doi:10.1016/S0957-4166(00)86322-3.
- ^ a b Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. (December 1999). "HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor". Proceedings of the National Academy of Sciences of the United States of America. 96 (25): 14228–14233. Bibcode:1999PNAS...9614228H. doi:10.1073/pnas.96.25.14228. PMC 24419. PMID 10588688.
- ^ "Properties of HU-308 ~ Formula C27H42O3". Pitt Quantum Repository. University of Pittsburgh Department of Chemistry.
- ^ "21 CFR — Schedules of controlled substances §1308.11 Schedule I." Archived from the original on 2009-08-27. Retrieved 2014-12-17.
- ^ "Chapter 893 - Drug abuse prevention and control". Florida Statutes.
