Onternabez: Difference between revisions
m Bot: Deprecating Template:Cite pmid and some minor fixations |
Slothwizard (talk | contribs) No edit summary Tags: Visual edit Mobile edit Mobile web edit |
||
| (235 intermediate revisions by 47 users not shown) | |||
| Line 1: | Line 1: | ||
{{short description|Cannabidiol-derivative drug}} |
|||
{{Drugbox |
{{Drugbox |
||
| Verifiedfields = changed |
| Verifiedfields = changed |
||
| Watchedfields = changed |
| Watchedfields = changed |
||
| class = [[CB2 receptor|CB<sub>2</sub> receptor agonist]] |
|||
| verifiedrevid = 426291380 |
| verifiedrevid = 426291380 |
||
| IUPAC_name = [( |
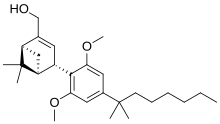
| IUPAC_name = [(1''S'',2''S'',5''S'')-2-[2,6-Dimethoxy-4-(2-methyloctan-2-yl)phenyl]-7,7-dimethyl-4-bicyclo[3.1.1]hept-3-enyl]methanol |
||
| image = |
| image = Onternabez.svg |
||
| |
| caption = |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> |
||
| ⚫ | |||
| pregnancy_US = <!-- A / B / C / D / X --> |
|||
| ⚫ | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> |
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> |
||
| legal_CA = |
| legal_CA = Schedule II |
||
| legal_UK = |
| legal_UK = Class B |
||
| legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> |
| legal_US = Unscheduled <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> <BR> |
||
| legal_US_comment = |
|||
| legal_status = |
| legal_status = Florida: Schedule I |
||
| routes_of_administration = |
| routes_of_administration = <!--Pharmacokinetic data--> |
||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
<!--Pharmacokinetic data--> |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
<!--Identifiers--> |
|||
| ⚫ | |||
| CAS_number = 256934-39-1 |
| CAS_number = 256934-39-1 |
||
| ⚫ | |||
| ATC_prefix = |
|||
| ⚫ | |||
| ⚫ | |||
| |
| ATC_prefix = None |
||
| ⚫ | |||
| PubChem = 11553430 |
|||
| ChEBI = 146244 |
|||
| synonyms = HU-308, HU308, PPP-003, ARDS-003 |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
||
| DrugBank = |
| DrugBank = |
||
| KEGG = D12305 |
|||
| ⚫ | |||
| ChemSpiderID = 8020425 |
|||
| ⚫ | |||
<!--Chemical data--> |
<!--Chemical data-->| C = 27 |
||
| H = 42 |
|||
| O = 3 |
|||
| molecular_weight = 415.63 g/mol |
|||
| smiles = CCCCCCC(C)(C)C1=CC(=C(C(=C1)OC) |
| smiles = CCCCCCC(C)(C)C1=CC(=C(C(=C1)OC)[C@H]2C=C([C@@H]3C[C@H]2C3(C)C)CO)OC |
||
| StdInChI = 1S/C27H42O3/c1-8-9-10-11-12-26(2,3)19-14-23(29-6)25(24(15-19)30-7)20-13-18(17-28)21-16-22(20)27(21,4)5/h13-15,20-22,28H,8-12,16-17H2,1-7H3/t20-,21-,22+/m0/s1 |
|||
| StdInChIKey = CFMRIVODIXTERW-FDFHNCONSA-N |
|||
}} |
}} |
||
'''HU-308''' is a |
'''Onternabez''' (also known as '''HU-308''', '''HU308''', '''PPP-003''', and '''ARDS-003''') is a [[Synthetic cannabinoids|synthetic cannabinoid]] that acts as a potent [[cannabinoid]] [[agonist]]. It is highly selective for the [[cannabinoid receptor type 2|cannabinoid-2 receptor]] (CB<sub>2</sub> receptor) subtype, with a selectivity more than 5,000 times greater for the CB<sub>2</sub> receptor than the [[CB1 receptor|CB<sub>1</sub> receptor]].<ref name="Mechoulam 1990">{{cite journal| vauthors = Mechoulam R, Lander N, Breuer A, Zahalka J |date=1990-04-11|title=Synthesis of the individual, pharmacologically distinct enantiomers of a tetrahydrocannabinol derivative|journal=Tetrahedron Asymmetry|volume=1|issue=5|language=en|pages=315–318|doi=10.1016/S0957-4166(00)86322-3|pmid=|pmc=|issn=|doi-access=free}}</ref><ref name="Hanus et al 1999">{{cite journal | vauthors = Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, Pertwee RG, Ross RA, Mechoulam R, Fride E | display-authors = 6 | title = HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 96 | issue = 25 | pages = 14228–14233 | date = December 1999 | pmid = 10588688 | pmc = 24419 | doi = 10.1073/pnas.96.25.14228 | doi-access = free | bibcode = 1999PNAS...9614228H }}</ref><ref>{{cite web |title=Properties of HU-308 ~ Formula C27H42O3 |url=http://pqr.pitt.edu/mol/CFMRIVODIXTERW-BHIFYINESA-N |website=Pitt Quantum Repository |publisher=University of Pittsburgh Department of Chemistry}}</ref> The [[chemical synthesis|synthesis]] and characterization of onternabez took place in the laboratory of [[Raphael Mechoulam]] at the [[Hebrew University of Jerusalem]] (the HU in HU-308) in the late 1990s. The [[pinene]] dimethoxy-DMH-CBD derivative onternabez was identified as a potent peripheral CB<sub>2</sub>-selective agonist in ''in vitro'' and animal studies in 1990<ref name="Mechoulam 1990" /> and 1999.<ref name="Hanus et al 1999" /> |
||
</ref> The [[chemical synthesis|synthesis]] and characterization took place in the laboratory of Prof. [[Raphael Mechoulam|Mechoulam]] at the [[Hebrew University of Jerusalem]] in the late 1990s. It has [[analgesic]] effects,<ref>{{Cite journal |
|||
| last1 = Labuda | first1 = C. |
|||
| last2 = Koblish | first2 = M. |
|||
| last3 = Little | first3 = P. |
|||
| title = Cannabinoid CB2 receptor agonist activity in the hindpaw incision model of postoperative pain |
|||
| journal = European Journal of Pharmacology |
|||
| volume = 527 |
|||
| issue = 1–3 |
|||
| pages = 172–174 |
|||
| year = 2005 |
|||
| pmid = 16316653 |
|||
| doi = 10.1016/j.ejphar.2005.10.020 |
|||
}}</ref> promotes [[cell proliferation|proliferation]] of [[neural stem cells]],<ref>{{Cite journal |author= Palazuelos, J., ''et al.'' |title= Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation |journal= The FASEB journal : official publication of the Federation of American Societies for Experimental Biology |volume= 20 |issue= 13 |pages= 2405–2407 |year= 2006 |pmid= 17015409 |doi= 10.1096/fj.06-6164fje }} |
|||
</ref> and protects both liver and blood vessel tissues against [[oxidative stress]] via inhibition of [[TNF-α]].<ref>{{Cite journal |
|||
| author= Rajesh | first= M. |
|||
| last2= Pan | first2= H. |
|||
| last3= Mukhopadhyay | first3= P. |
|||
| last4= Batkai | first4= S. |
|||
| last5= Osei-Hyiaman | first5= D. |
|||
| last6= Hasko | first6= G. |
|||
| last7= Liaudet | first7= L. |
|||
| last8= Gao | first8= B. |
|||
| last9= Pacher | first9= P. |title= Pivotal Advance: Cannabinoid-2 receptor agonist HU-308 protects against hepatic ischemia/reperfusion injury by attenuating oxidative stress, inflammatory response, and apoptosis |journal= Journal of leukocyte biology |volume= 82 |issue= 6 |pages= 1382–1389 |year= 2007 |pmid= 17652447 |doi= 10.1189/jlb.0307180 |pmc= 2225476 }} |
|||
</ref><ref>{{Cite journal |author= Rajesh, M., ''et al.'' |title= CB2-receptor stimulation attenuates TNF-α-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion |journal= American journal of physiology. Heart and circulatory physiology |volume= 293 |issue= 4 |pages= H2210–H2218 |year= 2007 |pmid= 17660390 |doi= 10.1152/ajpheart.00688.2007 |pmc= 2229632 }} |
|||
</ref> |
|||
==Legal status== |
==Legal status== |
||
===United States=== |
|||
HU-308 is not scheduled at the federal level in the [[United States]].<ref name="PART 1308 — SCHEDULES OF CONTROLLED SUBSTANCES - 1308.11 Schedule I">[http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm 21 CFR — SCHEDULES OF CONTROLLED SUBSTANCES §1308.11 Schedule I.]</ref> |
|||
Onternabez is non-psychoactive and not scheduled at the federal level in the United States.<ref>{{Cite web |url=http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm |title=21 CFR — Schedules of controlled substances §1308.11 Schedule I. |access-date=2014-12-17 |archive-date=2009-08-27 |archive-url=https://web.archive.org/web/20090827043725/http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm |url-status=dead }}</ref> It is a Schedule I [[controlled substance]] in the state of [[Florida]] making it illegal to buy, sell, or possess there.<ref>{{cite web | url = http://leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0800-0899/0893/0893.html | work = Florida Statutes | title = Chapter 893 - Drug abuse prevention and control }}</ref> |
|||
====Florida==== |
|||
"HU-308 ([(1R,2R,5R)-2-[2,6-dimethoxy-4-(2-methyloctan-2-yl)phenyl]-7,7-dimethyl-4-bicyclo[3.1.1]hept-3-enyl] methanol)" is a Schedule I [[controlled substance]] in the state of [[Florida]] making it illegal to buy, sell, or possess in Florida.<ref name="Florida Statutes - Chapter 893 - DRUG ABUSE PREVENTION AND CONTROL">[http://leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0800-0899/0893/0893.html Florida Statutes - Chapter 893 - DRUG ABUSE PREVENTION AND CONTROL]</ref> |
|||
==See also== |
== See also == |
||
* [[Cannabidiol dimethyl ether]] |
|||
* [[Cannabidiol diacetate]] |
|||
* [[HU-210]] |
* [[HU-210]] |
||
* [[HU-320]] |
* [[HU-320]] |
||
* [[HU-211]] |
|||
* [[Nabilone]] |
|||
* [[CP 47,497]] |
|||
==References== |
== References == |
||
{{reflist}} |
{{reflist}} |
||
{{Cannabinoids}} |
{{Cannabinoids}} |
||
[[Category: |
[[Category:Primary alcohols]] |
||
[[Category:Alcohols]] |
|||
[[Category:Phenol ethers]] |
[[Category:Phenol ethers]] |
||
[[Category:HU cannabinoids]] |
[[Category:HU cannabinoids]] |
||
[[Category:Designer drugs]] |
[[Category:Designer drugs]] |
||
[[Category:CB2 receptor agonists]] |
[[Category:CB2 receptor agonists]] |
||
{{cannabinoid-stub}} |
|||
Latest revision as of 08:24, 16 October 2024
 | |
| Clinical data | |
|---|---|
| Other names | HU-308, HU308, PPP-003, ARDS-003 |
| Drug class | CB2 receptor agonist |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Liver |
| Excretion | Kidneys |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H42O3 |
| Molar mass | 414.630 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Onternabez (also known as HU-308, HU308, PPP-003, and ARDS-003) is a synthetic cannabinoid that acts as a potent cannabinoid agonist. It is highly selective for the cannabinoid-2 receptor (CB2 receptor) subtype, with a selectivity more than 5,000 times greater for the CB2 receptor than the CB1 receptor.[1][2][3] The synthesis and characterization of onternabez took place in the laboratory of Raphael Mechoulam at the Hebrew University of Jerusalem (the HU in HU-308) in the late 1990s. The pinene dimethoxy-DMH-CBD derivative onternabez was identified as a potent peripheral CB2-selective agonist in in vitro and animal studies in 1990[1] and 1999.[2]
Legal status
[edit]Onternabez is non-psychoactive and not scheduled at the federal level in the United States.[4] It is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess there.[5]
See also
[edit]References
[edit]- ^ a b Mechoulam R, Lander N, Breuer A, Zahalka J (1990-04-11). "Synthesis of the individual, pharmacologically distinct enantiomers of a tetrahydrocannabinol derivative". Tetrahedron Asymmetry. 1 (5): 315–318. doi:10.1016/S0957-4166(00)86322-3.
- ^ a b Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, et al. (December 1999). "HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor". Proceedings of the National Academy of Sciences of the United States of America. 96 (25): 14228–14233. Bibcode:1999PNAS...9614228H. doi:10.1073/pnas.96.25.14228. PMC 24419. PMID 10588688.
- ^ "Properties of HU-308 ~ Formula C27H42O3". Pitt Quantum Repository. University of Pittsburgh Department of Chemistry.
- ^ "21 CFR — Schedules of controlled substances §1308.11 Schedule I." Archived from the original on 2009-08-27. Retrieved 2014-12-17.
- ^ "Chapter 893 - Drug abuse prevention and control". Florida Statutes.
